Acids and Bases
Chapter 5: Acids, Bases and Salts
Many acids and bases occur naturally in nature, such as citric acid in fruits like orange, lemon, etc, tartaric acid in tamarind, malic acid in apples, and lactic acid in milk and milk products, hydrochloric acid in gastric juices.
Similarly, many bases are found such as lime water. We use many of these acids in our day-to-day life, such as vinegar or acetic acid in the kitchen, boric acid for laundry, baking soda for the purpose of cooking, washing soda for cleaning, etc.
Acid and bases
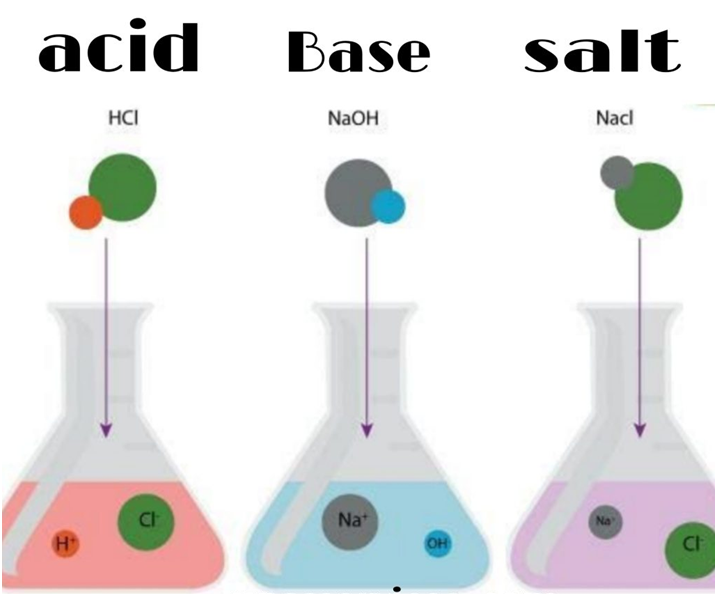
Acids and bases are popular chemicals which interact with each other resulting in the formation of salt and water. The word acid comes from a Latin word ‘acere’ which means ‘sour’.
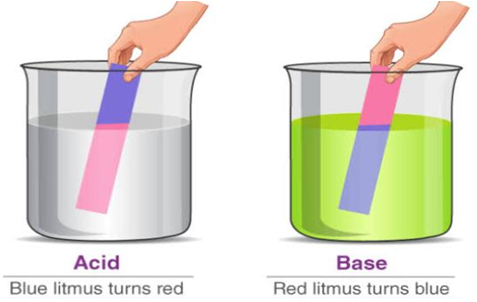
Natural Indicators Around Us
Natural indicators around us

Some examples of natural indicators are red cabbage, turmeric, grape juice, turnip skin, curry powder, cherries, beetroots, onion, tomato, etc. Some flowers like hydrangeas can determine the acidity or basicity of the soil.
Neutralisation
Neutralization
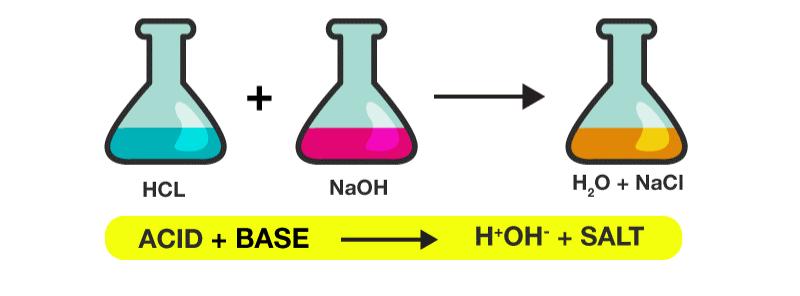
In chemistry, neutralization or neutralization (see spelling differences) is a chemical reaction in which acid and a base react quantitatively with each other. In a reaction in water, neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution. The pH of the neutralized solution depends on the acid strength of the reactants.
Neutralisations in Everyday Life.
Neutralization in everyday life
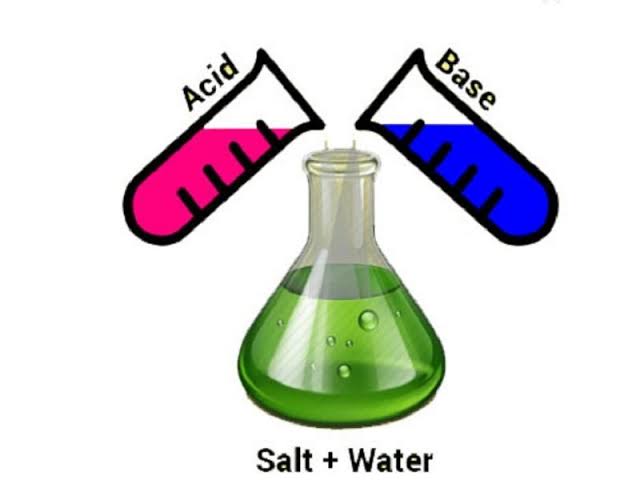
The reaction between an acid and a base is called neutralisation. In everyday life, it is employed in different applications. For example, it is used in the neutralisation of stomach acidity, in the prevention of tooth decay, neutralising the soil, in the treatment of ant’s bites, etc

 Param Publication
Param Publication
 Grow Career Publication
Grow Career Publication
