1. Photoelectric Effect
- Books Name
- Physics Book Part l and ll
- Publication
- Grow Career Publication
- Course
- CBSE Class 12
- Subject
- Physics
Chapter 11: Dual nature of radiation and matter

Photoelectric Effect
ELECTRON EMISSION
However, the free electrons cannot normally escape out of the metal surface. If an electron attempts to come out of the metal, the metal surface acquires a positive charge and pulls the electron back to the metal. The free electron is thus held inside the metal surface by the attractive forces of the ions. Consequently, the electron can come out of the metal surface only if it has got sufficient energy to over come the attractive pull. A certain minimum amount of energy is required to be given to an electron to pull it out from the surface of the metal. One electron volt is the energy gained by an electron when it has been accelerated by a potential difference of 1 volt, so
That 1 eV = 1.602 ×10–19 J.

PHOTOELECTRIC EFFECT
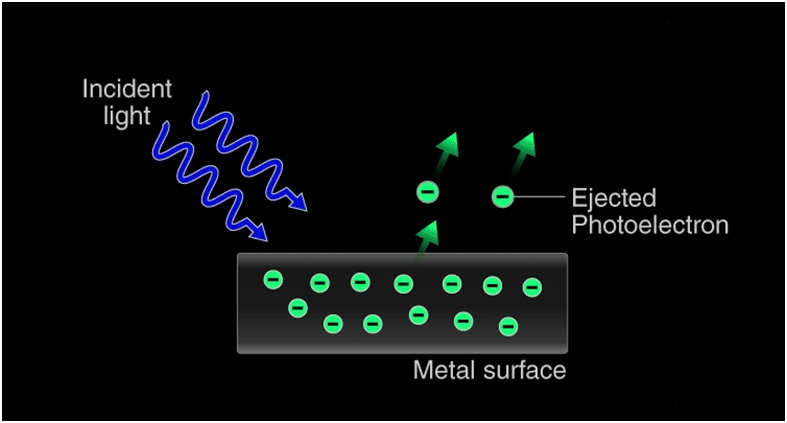
The photoelectric effect occurs because the electrons at the surface of the metal tend to absorb energy from the incident light and use it to overcome the attractive forces that bind them to the metallic nuclei. An illustration detailing the emission of photoelectrons as a result of the photoelectric effect is provided below.
The Concept of Photons
The photoelectric effect cannot be explained by considering light as a wave. However, this phenomenon can be explained by the particle nature of light, in which light can be visualized as a stream of particles of electromagnetic energy. These ‘particles’ of light are called photons. The energy held by a photon is related to the frequency of the light via Planck’s equation:
![]()
Where,
E denotes the energy of the photon
H is Planck’s constant
![]()
C is the speed of light (in a vacuum)
Λ is the wavelength of the light
Therefore, the relationship between the energy of the photon and the kinetic energy of the emitted photoelectron can be written as.
![]()
![]()
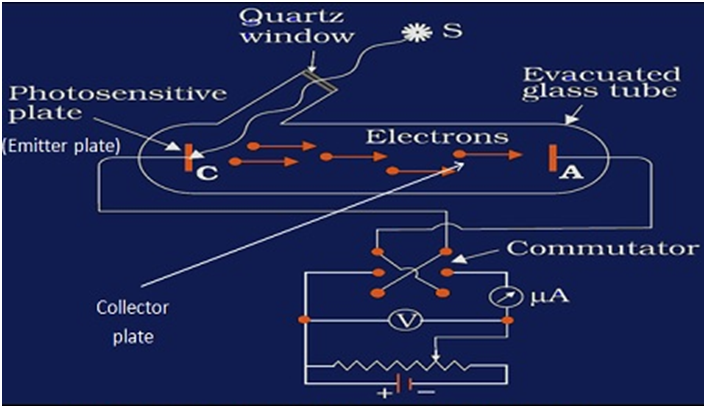
EXPERIMENTAL STUDY OF PHOTOELECTRIC EFFECT
- Evacuated tube consist of photosensitive plate (emitter) and the metal plate (collector), so that electrons could freely flow from emitter to collector without any air resistance
- Photosensitive plate (emitter) to absorb visible light and emit electrons
- Metal plate (collector) to receive electrons emitted from the emitter, thus constituting a photoelectric current flowfrom collector plate to the emitter plate (opposite to the flow of electrons)
- Monochromatic light of short wavelength (meaning high frequency)
- Battery to accelerate emitted electrons through a potential difference
- Voltmeter to measure the potential difference between the emitter and the collector plates due to photoelectric current flow
- Ammeter to measure the value of photoelectric current.
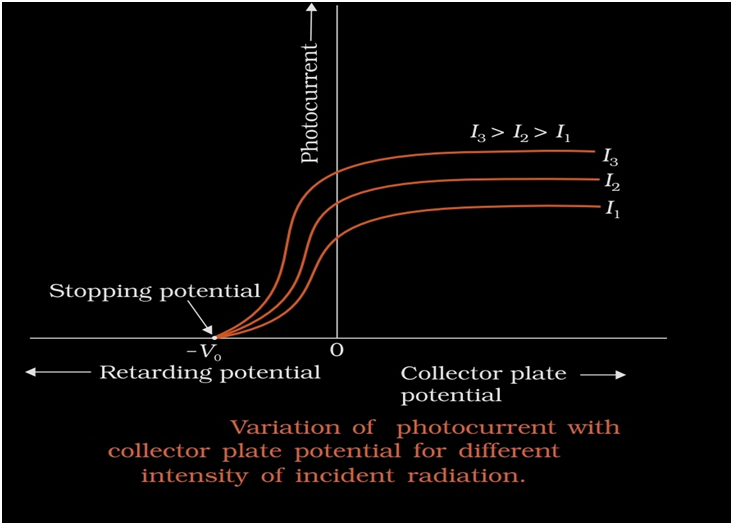
Effect of potential on photoelectric current
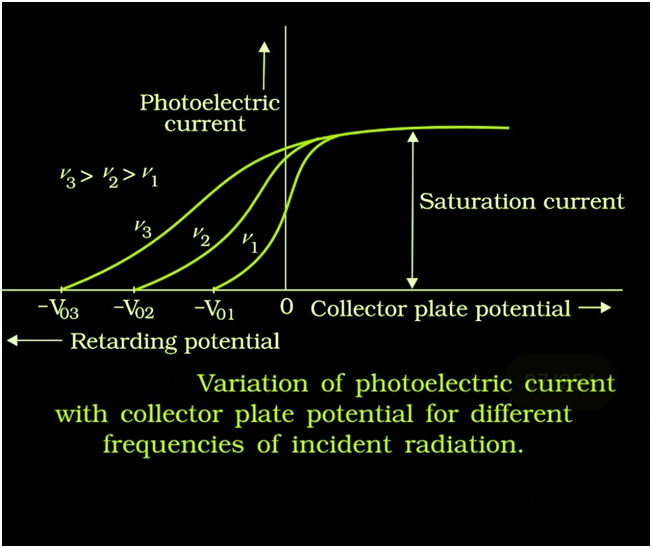
Effect of frequency of incident radiation on stopping
EINSTEIN’S PHOTOELECTRIC EQUATION: ENERGY QUANTUM OF RADIATION
Einstein resolved this problem using Planck’s revolutionary idea that light was a particle. The energy carried by each particle of light (called quanta or photon) is dependent on the light’s frequency (ν) as shown:
E = hν
Where h = Planck’s constant = 6.6261 × 10-34 J.
A part of this energy is used to remove the electron from the metal atom’s grasp and the rest is given to the ejected electron as kinetic energy. Electrons emitted from underneath the metal surface lose some kinetic energy during the collision. But the surface electrons carry all the kinetic energy imparted by the photon and have the maximum kinetic energy.
mathematically :
Energy of photon = energy required to eject an electron (work function) + Maximum kinetic energy of the electron
E = W + KE
Hv = W + KE
KE = hv – w
The energy of a photon with this frequency must be the work function of the metal.
W = hv0
The, Maximum kinetic energy equation,
KE = 1/2mv2max=hv–hv0
1/2mv2max=h(v−v0)
Vmax is the maximum kinetic energy of the electron. It is calculated experimentally using the stopping potential. Please read our article on Lenard’s observations to understand this part.
ev0 = 1/2mv2max
2. Photon
- Books Name
- Physics Book Part l and ll
- Publication
- Grow Career Publication
- Course
- CBSE Class 12
- Subject
- Physics
PARTICLE NATURE OF LIGHT: THE PHOTON
Photoelectric effect thus gave evidence to the strange fact that light in
interaction with matter behaved as if it was made of quanta or packets of
energy, each of energy hv.
Is the light quantum of energy to be associated with a particle? Einstein
arrived at the important result, that the light quantum can also be
associated with momentum (hv/c ). A definite value of energy as well as
momentum is a strong sign that the light quantum can be associated
with a particle. This particle was later named photon.
Wave Nature of Matter
The wave nature of matter is one of the most counter-intuitive concepts in Physics. You have seen examples of both particle nature of light and wave nature of light. You know about the Photoelectric effect due to Albert Einstein’s courtesy. In the photoelectric effect, the electrons and photons exhibit the properties of a particle, just like a billiard ball.
De Broglie’s Equation
Light and radiation are both particles and waves, according to De Broglie’s hypothesis, thus matter must also have a particle and wave character. Wave theory was born as a result of the de Broglie connection.
De Broglie’s equation is given as:
Λ = h ⁄ p = h ⁄ (mv)
Where,
Λ is the wavelength of particle,
P is the momentum of a particle,
H is the Planck’s constant,
M is the mass of particle, &
V is the velocity of the particle.
De Broglie’s Hypothesis
The momentum of a photon having energy E is given as:
P = E ⁄ c
The speed of light in a vacuum is represented by the letter c.≥
Planck’s idea states that the energy of a photon is determined by its frequency and wavelength.
E = h v = h c ⁄ λ
The energy should be equal, implying:
H c ⁄ λ = p c
Λ = h ⁄ p
De Broglie concluded that the aforementioned relationship should apply to particles as well. P=mv is the momentum of a particle with mass m moving at a speed of v. As a result, it must have a wavelength of
Λ = h ⁄ p = h ⁄ (mv)
DAVISSON AND GERMER EXPERIMENT
The experiment is performed by varying the accelerating voltage from 44 V to 68 V. A strong peak observed in the intensity (I) of the scattered electron for an accelerating voltage of 54 V at a scattering angle 50º. The appearance of the peak in a particular direction is due to the constructive interference of electrons scattered from different layers of the regularly spaced atoms of the crystals. From the electron diffraction theory, the wavelength of matter waves producing maxima at 50º is calculated to be λ= 0.165 nm.
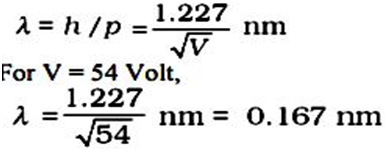
Thus, there is an excellent agreement between the theoretical value and the experimentally obtained value of de Broglie wavelength. Davisson- Germer experiment thus strikingly confirms the wave nature of electrons, particles in general and the de Broglie relation.

 Grow Career Publication
Grow Career Publication
