1. Atomic Masses and Composition of Nucleus
- Books Name
- Physics Book Part l and ll
- Publication
- Grow Career Publication
- Course
- CBSE Class 12
- Subject
- Physics
Chapter 13: Nuclei

Atomic Masses and Composition of Nucleus
Nucleus of an Atom – atomic mass
An atom is tiny and therefore its mass is also proportionally minute. A regular unit of mass such as a Kilogram (Kg) cannot be used to weigh something as small as an atom and to address this issue, scientists have created a new unit of mass. It is called the Atomic Mass Unit (u).
1 u = one atom of C-12/ 12 = 1.992647 10-26/ 12 kg
1 u = 1.660539 10-27 kg
Nucleus of an Atom – Composition
The Nucleus of an atom consists of a tightly packed arrangement of protons and neutrons. These are the two heavy particles in an atom and hence 99.9% of the mass is concentrated in the nucleus.
SIZE OF THE NUCLEUS
- The alpha particles got deflected because of repulsion with the nucleus as alpha particles are positively charged.They get repelled because they are both positively charged.
- Very small number of alpha particles got deflected proving that nucleus is very small in size.
- It was found that the radius of a nucleus (R) of mass number A is given as :-
- R=R0A1/3 where A = mass number and R0=constant.
- Volume of a nucleus is ∝to the mass number.
- V =(4/3)πR3 , Also R ∝(A)1/3
- R3∝A
- Therefore, V ∝ R3∝
- Density of nucleus is independent of mass number.
Mass – Energy
Einstein showed from his theory of special relativity that it is necessary to treat mass as another form of energy. Before the advent of this theory of special relativity it was presumed that mass and energy were conserved separately in a reaction. Einstein gave the famous mass-energy equivalence relation
E = mc2
Nuclear binding energy
Importance of nuclear binding energy describes how strongly nucleons are bound to each other. By determining its value we will come to know whether the neutrons and protons are tightly or loosely bound to each other.

If nuclear binding energy is high -> high amount of energy is needed to separate the nucleons this means nucleus is very stable.
Energy required holding neutrons and protons together therefore keeps the nucleus intact.
It can also be defined as the energy needed to separate the nucleons from each other.
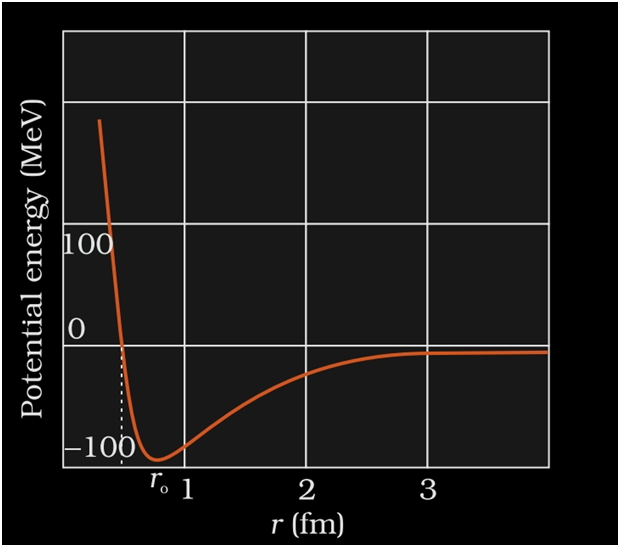
NUCLEAR FORCE
- It is the strong attractive force that binds the nucleons together.
- When the nuclear force is compared to other forces of nature like gravitational or coulomb’s force etc.it is the strongest of all the forces.
- As protons are positively charged they repel each other.This force of repulsion is given by Coulomb’s force of repulsion.
- This nuclear force is stronger than the coulomb’s force so it overcomes the force of repulsion.
- This is the reason neutrons and protons are held together inside the nucleus.
- The force with which the nucleons are bound together is known as nuclear force.
Radioactivity
Nuclear instability, an atom’s nucleus exhibits the phenomenon of Radioactivity. Energy is lost due to radiation that is emitted out of the unstable nucleus of an atom. Two forces, namely the force of repulsion that is electrostatic and the powerful forces of attraction of the nucleus keep the nucleus together.
- Gamma Decay (Photons having high energy are emitted)
- Beta Decay (Emission consists of Electrons)
- Alpha Decay (Emission consists of Helium nucleus)
Alpha decay
In alpha decay α particles are emitted.Daughter nucleus is formed from the parent nucleus.
The atomic number decreases by 2 and Mass number increases by 4.
It is a very spontaneous process and happens on its own.
It occurs only in radionuclides.
For example:-
23892U (unstable) --> 90234Th + 24He
(Uranium (U) is known as parent nucleus and Thorium (Th) is known as daughter nucleus).
24He is α particle.
General form of alpha decay: -AZX (parent) à(A-4) (Z-2) Y + 24He
Where Y is daughter nucleus.
Q-value of alpha decay
Q-value is a parameter or a characteristic of a nuclear reaction which describes whether the reaction can take place or not.
Q-value is defined as difference in the initial mass energy and final mass energy of decayed products.
Consider the general equation:-
AZX (parent) -->(A-4) (Z-2) Y + 24
Initial rest mass energy Ui =[m (AZX) - Zme]c2
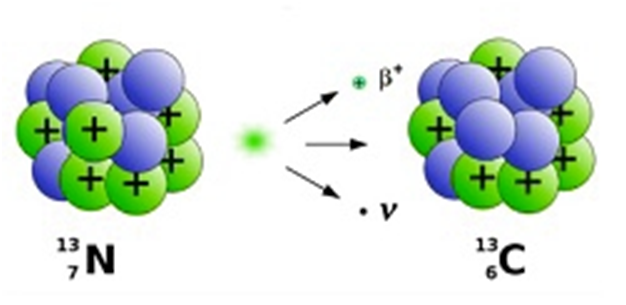
Beta decay
In case of beta decay either electron or positron is emitted.
Mass number remains the same.
β−decay --> Electron is emitted.
Atomic number increases by 1.
For example:- 3215P à1632S + e- + ̅ν where ̅ν =anti-neutrino
Q-value for β−decay:-
AZXàA (Z+1) Y + e-+ ̅ν
Initial rest mass energy Ui = [m (AZX) - Zme]c2
Gamma decay
In Gamma decay γ rays are emitted.γ rays are electromagnetic waves with short wavelength. Most of the daughter nuclei of alpha decay and beta decay are in excited state. As a result they are unstable.When the daughter nuclides try to transit from excited state to ground state they emit radiations.
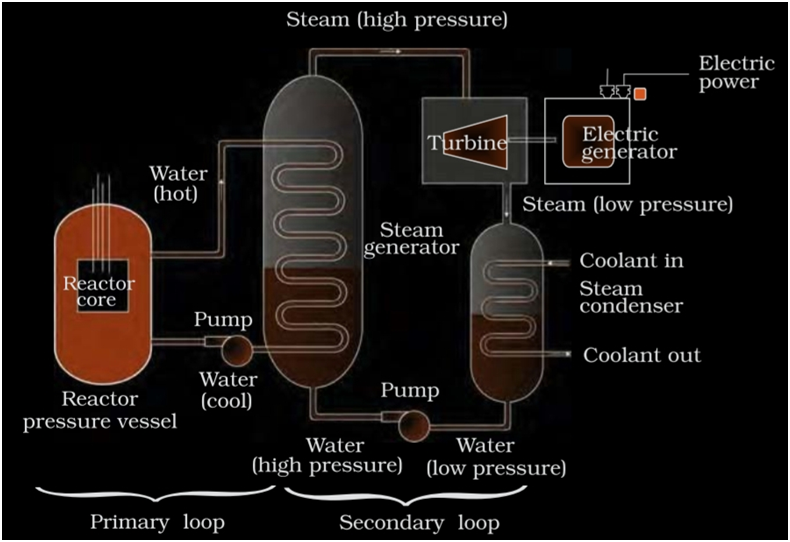
- Nuclear Energy
Electric energy can be harnessed from nuclear energy. Nuclear energy is the energy that holds together the nuclei of atoms. Nuclear energy is obtained from nucleusby either Breaking of heavy nucleus into 2 relatively lighter nuclei known as nuclear fission or by Combining 2 lighter nuclei to form a heavy nucleus known as nuclear fusion.

 Grow Career Publication
Grow Career Publication
