Hydrogen
- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
HYDROGEN
- Hydrogen is the lightest element known since it has an atomic mass of 1.0079
- Hydrogen resembles Group 1 elements
- Hydrogen also resembles elements of group 17 (Halogens)
- (H+) is of size ~10–5 Ao. This is extremely small as compared to normal atomic and ionic sizes of ~1 Ao. As a consequence, H+ does not exist freely and is always associated with other atoms or molecules.
- Thus, it is unique in behaviour and is, therefore, best placed separately in the periodic table
OCCURRENCE
It is the most abundant element in the universe (70% of the total mass of the universe). It is much less abundant (0.15% by mass) in the earth’s atmosphere. Of course, in the combined form it constitutes 15.4% of the earth's crust and the oceans

H is the predominant form 99.98%
D is (0.0156% of total)
T. (one atom per 1018 atoms)
Tritium is radioactive and emits low energy ß particles (t½, 12.33 years).
Preparation of Hydrogen Gas
Laboratory Preparation
(i) Zn + 2H+ ® Zn2+ + H2
(ii) Zn + 2NaOH ® Na2ZnO2(Sodium zincate) + H2
Commercial Production
(i) Electrolysis of acidified water using platinum electrodes gives hydrogen.
(ii) High purity (>99.95%) hydrogen is obtained by electrolysing warm aqueous barium hydroxide solution between nickel electrodes
(iii) By the electrolysis of brine solution.
anode: 2Cl–(aq) ® Cl2(g) + 2e–
cathode: 2H2O (l) + 2e– ® H2(g) + 2OH–(aq)
2NaCl (aq) + 2H2O(l) ®Cl2(g) + H2(g) + 2NaOH(aq)
Water gas: - is the mixture of CO and H2
Water-gas shift reaction
C(s) + H2O 1000°CNi CO + H2 CO(g) + H2O 400°Ciron cromate
CO + H2 CO(g) + H2O 400°Ciron cromate CO2 + H2
CO2 + H2
PROPERTIES OF HYDROGEN
Physical Properties
- Hydrogen gas is a colourless, odourless, tasteless, highly combustible gas.
- It is lighter than air and insoluble in water.
Chemical Properties
- The H–H bond dissociation enthalpy is the highest for a single bond between two atoms of any element. it is relatively inert at room temperature.
- Since its orbital is incomplete with 1s1 electronic configuration, it does combine with almost all the elements. It accomplishes reactions by
- loss of the only electron to give H+,
- gain of an electron to form H–, and
(iii) sharing electrons to form a single covalent bond
(i) Reaction with halogens
H2(g) + X2(g) ® 2HX(g), While the reaction with fluorine occurs even in the dark, with iodine it requires a catalyst.
(ii)Reaction with oxygen
2H2(g) + O2 (g) ® 2H2O(l); DH = –285.9 kJ mol–1
(iii) Reaction with nitrogen
3H2(g) + N2(g) 400°Ciron 2NH3(g)
2NH3(g)
(iv) Reactions with metals:
it forms corresponding hydrides H2(g) +2M(g) ® 2MH(s); where M is an alkali metal
(v) Reactions with metal ions and metal oxides:
It reduces some metal ions in aqueous solution and oxides of metals (less active than iron) into corresponding metals.
H2(g) + Pd++(aq) ® Pd(s) + 2H+(aq)
yH2(g) + MxOy(s) ® xM(s) + yH2O(l)
(vi) Reactions with organic compounds:
It reacts with many organic compounds in the presence of catalysts to give useful hydrogenated products of commercial importance
USES OF HYDROGEN GAS
1. The single largest use of hydrogen is in the synthesis of ammonia which is used in the manufacture of nitric acid and nitrogenous fertilizers.
2. Hydrogen is used in the manufacture of vanaspati fat by the hydrogenation of polyunsaturated vegetable oils like soybean, cotton seeds etc.
3. It is used in the manufacture of bulk organic chemicals, particularly methanol.
4. It is widely used for the manufacture of metal hydrides.
5. It is used for the preparation of hydrogen chloride, a highly useful chemical.
6. In metallurgical processes, it is used to reduce heavy metal oxides to metals.
7. Atomic hydrogen and oxy-hydrogen torches find use for cutting and welding purposes. Atomic hydrogen atoms (produced by dissociation of hydrogen with the help of an electric arc) are allowed to recombine on the surface to be welded to generate the temperature of 4000 K.
8. It is used as a rocket fuel in space research.
9. Hydrogen is used in fuel cells for generating electrical energy. It has many advantages over the conventional fossil fuels and electric power. It does not produce any pollution and releases greater energy per unit mass of fuel in comparison to gasoline and other fuels.
HYDRIDES
Hydrogen, under certain reaction conditions, combines with almost all elements, except noble gases, to form binary compounds, called hydrides. If ‘E’ is the symbol of an element then hydride can be expressed as EHX (e.g., MgH2) or EXHY (e.g., B2H6)
The hydrides are classified into three categories:

Water
- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
WATER
Human body has about 65% water and some plants have as much as 95% water. It is a crucial compound for the survival of all life forms.
Physical Properties of Water
- It is a colourless and tasteless liquid.
- The presence of extensive hydrogen bonding between water molecules leads to high freezing point, high boiling point, high heat of vaporisation and high heat of fusion in comparison to H2S and H2Se.
- Water has a higher specific heat, thermal conductivity, surface tension, dipole moment and dielectric constant, etc.
STRUCTURE OF WATER
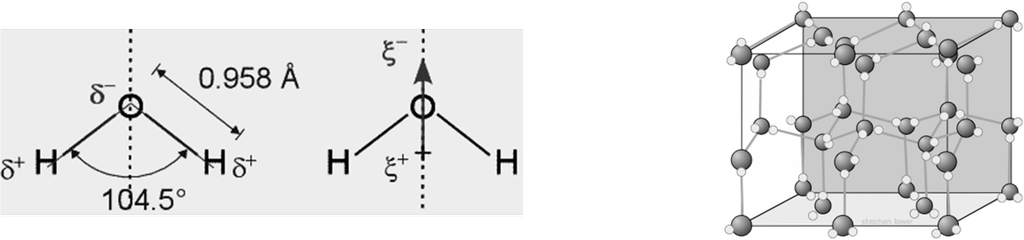
CHEMICAL PROPERTIES OF WATER
(1) Amphoteric Nature:
H2O(l) + NH3(aq) ⇌ OH-(aq) + NH4+(aq)
H2O(l) + H2S(aq) ⇌ H3O+(aq) + HS-(aq)
The auto-protolysis (self-ionization) of water takes place as follows:
H2O(l) + H2O(l) ⇌ H3O+(aq) + OH-(aq)
(2) Redox Reactions: Water is easily reduced to hydrogen by highly electropositive metals.
2H2O(l) + 2Na(s) → 2NaOH(aq) + H2(g)
With fluorine also it is oxidised to O2.
2F2(g) + 2H2O(l) ® 4H+ (aq) + 4F–(aq) + O2(g)
(3) Hydrolysis Reaction: Due to high dielectric constant, it has a very strong hydrating tendency..
P4O10(s) + 6H2O(l) → 4H3PO4(aq)
SiCl4(l) + 2H2O(l) → SiO2(s) + 4HCl(aq)
(4) Hydrates Formation:
(i) coordinated water e.g. [Cr(H2O)6]Cl3
(ii) interstitial water e.g., BaCl2.2H2O
(iii) hydrogen-bonded water eg. [Cu(H2O)4]SO4.H2O
HARD AND SOFT WATER
- Temporary hardness,
- Permanent hardness.
Temporary Hardness due to the presence of magnesium and calcium bicarbonates. It can be removed by:
(i) Boiling:
Mg(HCO3)2 ⎯ heating® Mg(OH)2 ↓ + 2CO2 ↑
Ca(HCO3)2 ⎯ heating® CaCO3 ↓ +H2O+ CO2 ↑
(ii) Clark’s method:
Ca(HCO3)2 + Ca(OH)2 ® 2CaCO3 ↓ +2H2O
Mg(HCO3)2 +2Ca(OH)2 ®2CaCO3¯ +Mg(OH)2¯ +2H2O
Permanent Hardness is due to the presence of soluble salts of magnesium and calcium in the form of chlorides and sulphates in water. Permanent hardness cannot be removed by boiling. It can be removed by the following methods.
(i) Treatment with washing soda (sodium carbonate):
MCl2 + Na2CO3 ® MCO3↓ + 2NaCl (M=Mg, Ca)
MSO4 + Na2CO3 ® MCO3↓ + Na2SO4
(ii) Calgon’s method: Sodium hexametaphosphate (Na6P6O18), commercially called ‘calgon’, when added to hard water, the following reactions take place.
Na6P6O18 ® 2Na+ +Na4P6O18-2 (M = Mg, Ca)
M+2 +Na4P6O18-2 ® Na2MP6O18-2 +2Na+ the complex anion keeps the Mg2+ and Ca2+ ions in solution
(iii) Ion-exchange method: sodium aluminium silicate (NaAlSiO4) can be written as NaZ. When this is added in hard water, exchange reactions take place.
2NaZ(s) +M+2(aq) ® MZ2(s) +2Na+(aq) (M=Mg, Ca)
Permutit/zeolite is said to be exhausted when all the sodium in it is used up. It is regenerated for further use by treating with an aqueous sodium chloride solution.
MZ2(s) +2NaCl(aq) →2NaZ(s) + MCl2
Hydrogen Peroxide (H2O2)
PREPARATION
(i) Acidifying barium peroxide and removing excess water by evaporation under reduced pressure gives hydrogen peroxide.
BaO2.8H2O(s) + H2SO4(aq) ® BaSO4(s) + H2O2(aq) + 8H2O(l)
(ii) Peroxodisulphate, obtained by electrolytic oxidation of acidified sulphate solutions at high current density, on hydrolysis yields hydrogen peroxide.
2HSO4-(aq) - electrolysis ® HO3SOOSO3H(aq) -hydrolysis ® 2HSO4-(aq) + 2H+(aq) + H2O2(aq)
(iii) Industrially it is prepared by the auto-oxidation of 2-alklylanthraquinols.
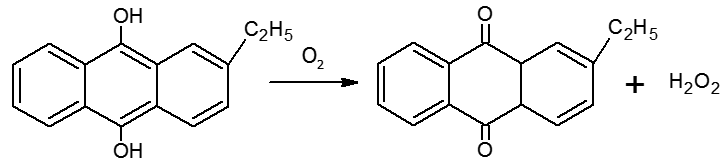
In this case 1% H2O2 is formed. It is extracted with water and concentrated to ~30% (by mass) by distillation under reduced pressure. It can be further concentrated to ~85% by careful distillation under low pressure. The remaining water can be frozen out to obtain pure H2O2
PHYSICAL PROPERTIES
In the pure state H2O2 is an almost colourless (very pale blue) liquid. H2O2 is miscible with water in all proportions and forms a hydrate H2O2.H2O (M.P. 221K). A 30% solution of H2O2 is marketed as ‘100 volume’ hydrogen peroxide. It means that one millilitre of 30% H2O2 solution will give 100 V of oxygen at STP. Commercially, it is marketed as 10 V, which means it contains 3% H2O2.
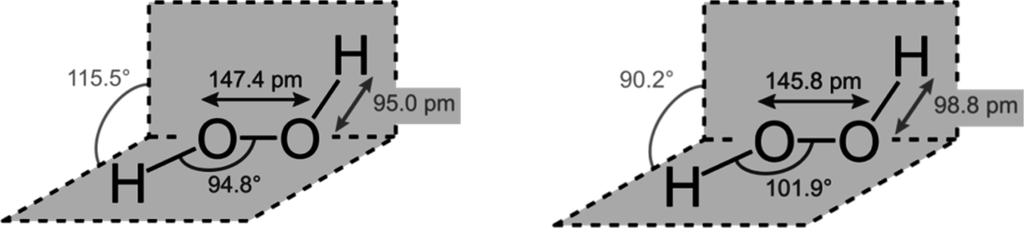
STRUCTURE
Hydrogen peroxide has a non-planar structure. The molecular dimensions in the gas phase and solid phase are shown:
CHEMICAL PROPERTIES
It acts as an oxidising as well as reducing agent in both acidic and alkaline media. Simple reactions are described below.
(i) Oxidising action in acidic medium
2Fe+2(aq) + 2H+(aq) +H2O2(aq) ® Fe+3(aq) + 2H2O
PbS(s) + 4H2O2(aq) ® PbSO4(s) + 4H2O
(ii) Reducing action in acidic medium
2MnO4-(aq) + 5H2O2(l) + 6H+ ® 2Mn2+(aq) + 5O2(g) + 8H2O
HOCl + H2O2 ®H3O+ + Cl- + O2
(iii) Oxidising action in basic medium
2Fe+2 + H2O2 ® Fe+3 + 2OH-
Mn+2 + H2O2 ® Mn+4 + 2OH-
(iv) Reducing action in basic medium
I2 + H2O2 + 2OH- ® 2I- + 2H2O + O2
2MnO4- + 3H2O2 ® 2MnO2 +3O2 + 2H2O +2OH-
STORAGE
H2O2 decomposes slowly on exposure to light.
2H2O2(l) ® 2H2O(l) + O2(g)
In the presence of metal surfaces or traces of alkali (present in glass containers), the above reaction is catalysed. It is, therefore, stored in wax-lined glass or plastic vessels in dark. Urea can be added as a stabiliser. It is kept away from dust because dust can induce explosive decomposition of the compound.
Uses
Its wide scale use has led to tremendous increase in the industrial production of H2O2. Some of the uses are listed below:
(i) In daily life it is used as hair bleach and as a mild disinfectant. As an antiseptic it is sold as perhydrol.
(ii) It is used to manufacture chemicals like sodium perborate and per-carbonate, which are used in high quality detergents.
(iii) It is used in the synthesis of hydroquinone, tartaric acid and certain food products and pharmaceuticals (cephalosporin) etc.
(iv) It is employed in the industries as a bleaching agent for textiles, paper pulp, leather, oils, fats, etc.
(v) Nowadays it is also used in Environmental (Green) Chemistry. For example, in pollution control treatment of domestic and industrial effluents, oxidation of cyanides, restoration of aerobic conditions to sewage wastes, etc.
Heavy Water, D2O
It is extensively used as a moderator in nuclear reactors and in exchange reactions for the study of reaction mechanisms. It can be prepared by exhaustive electrolysis of water or as a by-product in some fertilizer industries.
It is used for the preparation of other deuterium compounds, for example:
CaC2 +D2O ® C2D2 + Ca(OD)2
SO3 + D2O ® D2SO4
Al4C3 + 12D2O ® 3CD4 + 4Al(OD)3
S-Block elements
- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
S-BLOCK ELEMENTS
Group I (or I A) Elements: (Alkali Metals)
The alkali metals show regular trends in their physical and chemical properties with the increasing atomic number. The atomic, physical and chemical properties of alkali metals are discussed below
Electronic Configuration:-All the alkali metals have one valence electron, ns1
Atomic and Ionic Radii:-
- Largest sizes in a particular period of the periodic table.
- With increase in atomic number, the atom becomes larger.
- The atomic and ionic radii of alkali metals increase on moving down the group
Ionisation Enthalpy
- The ionisation enthalpies of the alkali metals are considerably low and decrease down the group from Li to Cs.
- The second ionization enthalpies of alkali metals are very high.
Melting and boiling points: Alkali metals are soft and have low melting and boiling point.
Density: Densities of alkali metals are quite low as compared to other metals
Electropositive or metallic character: All the alkali metals are strongly electropositive or metallic in character
Oxidation states: All the alkali metals predominantly exhibit an oxidation state of +1 in their compounds
Characteristic flame colouration: All the alkali metals and their salts impart characteristic flame colouration
Photoelectric effect: Phenomenon of ejection of electrons when electromagnetic radiation of suitable frequency strikes metal surface is called photo-electric effect. Alkali metals exhibit photo-electric effect
Physical appearance:- All the alkali metals are silvery white, soft and light metals. They have low density which increases down the group from Li to Cs. However, potassium is lighter than sodium.
Reducing Nature: Alkali metals are strong reducing agents
Chemical Properties
The alkali metals are highly reactive due to their large size and low ionisation enthalpy. The reactivity of these metals increases down the group.
(i) Reactivity towards air: The alkali metals tarnish in dry air due to the formation of their oxides which in turn react with moisture to form hydroxides. They burn vigorously in oxygen forming oxides. Lithium forms monoxide, sodium forms peroxide, the other metals form superoxides. The superoxide O2 – ion is stable only in the presence of large cations such as K, Rb, and Cs.
4 Li + O2 →2 Li2O (oxide)
2 Na + O 2 →Na2O2 (peroxide)
M + O2 → MO2 (superoxide)(M = K, Rb, Cs).
(ii) Reactivity towards water: The alkali metals react with water to form hydroxide and hydrogen.
2M + 2H2O → 2 M+ + 2OH- + H2 (M = an alkali metal)
It may be noted that although lithium has most negative E0 value, its reaction with water is less vigorous than that of sodium which has the least negative E0 value among the alkali metals. This behaviour of lithium is attributed to its small size and very high hydration energy. Other metals of the group react explosively with water
(iii) Reactivity towards hydrogen: The alkali metals react with hydrogen at about 673K (lithium at 1073K) to form hydrides. All the alkali metal hydrides are ionic solids with high melting points. 2M + H2 → 2MH.
(iv) Reactivity towards halogens: The alkali metals readily react with halogens to form ionic halides. Lithium iodide is the most covalent in nature.
(v) Reducing nature: The alkali metals are strong reducing agents, lithium being the most and sodium the least powerful.
(vi) Solutions in liquid ammonia: The alkali metals dissolve in liquid ammonia giving deep blue solutions which are conducting in nature. M + (x + y)NH3 → [M(NH3) x]+ + [e(NH3)y]-
The blue colour of the solution is due to the ammoniated electron which absorbs energy in the visible region of light and thus imparts blue colour to the solution
Anomalous Properties of Lithium
The anomalous behaviour of lithium is due to the:
(i) Exceptionally small size of its atom and ion, and
(ii) High polarizing power (i.e.., charge/ radius ratio).
Points of Difference between Lithium and other Alkali Metals
- Lithium is much harder. Its M.P. and B.P. are higher than the other alkali metals.
- Lithium is least reactive but the strongest reducing agent among all the alkali metals. On combustion in air it forms mainly monoxide, Li2O and the nitride, Li3N unlike other alkali metals.
- LiCl is deliquescent and crystallizes as a hydrate, LiCl.2H2O whereas other alkali metal chlorides do not form hydrates.
- Lithium hydrogen carbonate is not obtained in the solid form while all other elements form solid hydrogen carbonates.
- Lithium unlike other alkali metals forms no ethynide on reaction with ethyne.
- Lithium nitrate when heated gives lithium oxide, Li2O, whereas other alkali metal nitrates decompose to give the corresponding nitrite.
- LiF and Li2O are comparatively much less soluble in water than the corresponding compounds of other alkali metals.
The similarity between lithium and magnesium is particularly striking and arises because of their similar sizes: atomic radii, Li = 152 pm, Mg = 160 pm; ionic radii: Li+ = 76 pm, Mg2+= 72 pm. The main points of similarity are:
- Both lithium and magnesium are harder and lighter than other elements in the respective groups.
- Lithium and magnesium react slowly with water. Their oxides and hydroxides are much less soluble and their hydroxides decompose on heating. Both form a nitride, Li3N and Mg3N2, by direct combination with nitrogen.
- The oxides, Li2O and MgO do not combine with excess oxygen to give any superoxide.
- The carbonates of lithium and magnesium decompose easily on heating to form the oxides and CO2. Solid hydrogen carbonates are not formed by lithium and magnesium.
- Both LiCl and MgCl2 are soluble in ethanol.
- Both LiCl and MgCl2 are deliquescent and crystallize from aqueous solution as hydrates, LiCl·2H2O and MgCl2·8H2O.
SOME IMPORTANT COMPOUNDS OF SODIUM
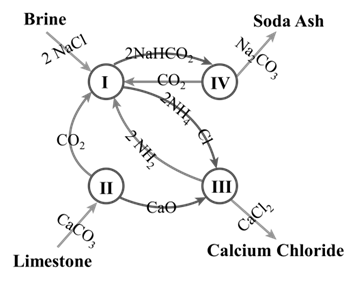
Sodium Carbonate (Washing Soda),Na2CO3·10H2O
Preparation: Solvay’s process.
The overall process is:
2 NaCl + CaCO3 → Na2CO3 + CaCl2
But it is done in following four steps
- NaCl + CO2 + NH3 + H2O → NaHCO3↓ + NH4Cl (I)
- CaCO3 → CO2 + CaO (II)
- 2NH4Cl + CaO → 2NH3 + CaCl2 + H2O (III)
 2NaHCO3 → Na2CO3 + H2O + CO2 (IV)
2NaHCO3 → Na2CO3 + H2O + CO2 (IV)
Properties: Sodium carbonate is a white crystalline solid which exists as a decahydrate, Na2CO3·10H2O. It is readily soluble in water. On heating, the decahydrate loses its water of crystallisation to form monohydrate. Above 373K, the monohydrate becomes completely anhydrous and changes to a white powder called soda ash.
Na2CO3.10H2O∆Na2CO3.H2O+9H2O Na2CO3.H2O>100 oCNa2CO3+H2O
Na2CO3.H2O>100 oCNa2CO3+H2O
Uses:
(i) It is used in water softening, laundering and cleaning.
(ii) It is used in the manufacture of glass, soap, borax and caustic soda.
(iii) It is used in paper, paints and textile industries.
(iv) It is an important laboratory reagent both in qualitative and quantitative analysis
Sodium Hydroxide (Caustic Soda), NaOH manufactured by the electrolysis of brine solution in the Castner –Kellner cell
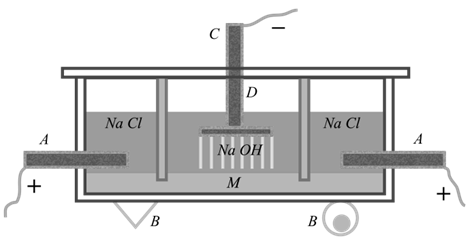

The cell is divided into three partitions. The graphite anode is fixed on the two outer partitions while the cathode is an iron rod which is fit in the central compartment. A layer of mercury, which serves an intermittent electrode, covers the bottom of the cell. Brine solution is placed in the outer compartments. The equation for the electrolytic process is as given below.
Outer Compartment:-The mercury layer acts as the cathode:-
NaCl® Na+ + Cl—
At Cathode:-Na+ + e- ® Na
At Anode: Cl— ® Cl +e- and Cl + Cl ® Cl2
the sodium, which is formed, dissolves in mercury to form an amalgam. It goes into the central compartment.
Central Compartment: - Here sodium amalgam acts as anode by induction. Sodium reacts with water to form NaOH and H2. Hydrogen gas is liberated at the cathode. The equations are as given below. 2Na/Hg + 2H2O ® 2NaOH + H2 + 2Hg
When the conc. of NaOH reaches 20%, it is removed and the solution is concentrated to get solid NaOH.
Group II ( or II A) Elements: (Alkali Earth Metals)
These (except beryllium) are known as alkaline earth metals. The first element beryllium differs from the rest of the members and shows diagonal relationship to aluminium.
Electronic Configuration: These elements have two electrons in the s orbital of the valence shell [noble gas] ns2.
ATOMIC AND IONIC RADII
Atomic and ionic radii of alkaline earth metals increases down the group and are smaller than the corresponding members of the alkali metals
IONISATION ENTHALPIES
The alkaline earth metals have low ionisation enthalpies
Since the atomic size increases down the group, their ionisation enthalpy decreases. Although IE1 values of alkaline earth metals are higher than those of alkali metals, the IE2 values of alkaline earth metals are much smaller than those of alkali metals
Melting and boiling points: Alkaline earth metals have higher melting and boiling points than the corresponding alkali metals
Electropositive or metallic character: The electropositive character increases down the group i.e., from Be to Ba but alkaline earth metals are not as strongly electropositive as the alkali metals
Oxidation states: All the alkaline earth metal exhibits an oxidation state of +2 in their compounds
HYDRATION ENTHALPIES
Like alkali metal ions, the hydration enthalpies of alkaline earth metal ions decrease with increase in ionic size down the group.
Be2+> Mg2+ > Ca2+ > Sr2+ > Ba2+
PHYSICAL APPEARANCE
The alkaline earth metals, in general, are silvery white, lustrous and relatively soft but harder than the alkali metals
Chemical Properties: The alkaline earth metals are less reactive than the alkali metals. The reactivity of these elements increases on going down the group.
(i) Reactivity towards air and water: Beryllium and magnesium are kinetically inert to oxygen and water because of the formation of an oxide film on their surface. However, powdered beryllium burns brilliantly on ignition in air to give BeO and Be3N2
(ii) Reactivity towards the halogens: All the alkaline earth metals combine with halogen at elevated temperatures forming their halides.
M + X 2 ® MX2 ( X = F, Cl, Br, l)
(iii) Reactivity towards hydrogen: All the elements except beryllium combine with hydrogen upon heating to form their hydrides,MH2. .BeH2, however, can be prepared by the reaction of BeCl2 with LiAlH4 as follows:
2BeCl 2 + LiAlH4 ® 2BeH2 + LiCl + AlCl3
(iv) Reactivity towards acids: The alkaline earth metals readily react with acids liberating hydrogen.
M + 2HCl ® MCl2 + H2
(v) Reducing nature: alkaline earth metals are strong reducing agents
(vi) Solutions in liquid ammonia: alkaline earth metals dissolve in liquid ammonia to give deep blue black solutions forming ammoniated ions.
M + (x+ y) NH3 ® [M(NH3) x]2+ + 2[e(NH3)x]-
COMPOUNDS OF ALKALI EARTH METALS
(i) Oxides and Hydroxides:
The alkaline earth metals burn in oxygen to form the monoxide, MO
(ii) Halides: Except for beryllium halides, all other halides of alkaline earth metals are ionic in nature. Beryllium chloride has a chain structure in the solid state as shown

(iii) Carbonates: Carbonates of alkaline earth metals are insoluble in water and can be precipitated by addition of a sodium or ammonium carbonate solution to a solution of a soluble salt of these metals. The solubility of carbonates in water decreases as the atomic number of the metal ion increases. All the carbonates decompose on heating to give carbon dioxide and the oxide. Beryllium carbonate is unstable and can be kept only in the atmosphere of CO2.
(iv) Sulphates: The sulphates of the alkaline earth metals are all white solids and stable to heat. BeSO4, and MgSO4 are readily soluble in water the solubility decreases from CaSO4 to BaSO4
ANOMALOUS BEHAVIOUR OF BERYLLIUM
Beryllium shoes anomalous behaviour as compared to magnesium and rest of the members. Further, it shows diagonal relationship to aluminium
(i) Beryllium has exceptionally small atomic and ionic sizes and thus does not compare well with other members of the group. Because of high ionisation enthalpy and small size it forms compounds which are largely covalent and get easily hydrolysed.
(ii) Beryllium does not exhibit coordination number more than four as in its valence shells there are only four orbitals. The remaining members of the group can have a coordination number of six by making use of d-orbitals
(iii) The oxide and hydroxide of beryllium, unlike the hydroxides of other elements in the group, are amphoteric in nature.
Diagonal Relationship between Beryllium and Aluminium
The ionic radius of Be2+ is estimated to be 31 pm; the charge/radius ratio is nearly the same as that of the Al3+ ion.:
(i) Like aluminium, beryllium is not readily attacked by acids because of the presence of an oxide film on the surface of the metal.
(ii) Beryllium hydroxide dissolves in excess of alkali to give a beryllate ion, Be(OH)4]2– just as aluminium hydroxide gives aluminate ion, [Al(OH)4]–.
(iii) The chlorides of both beryllium and Aluminium has Cl– bridged chloride structure in vapour phase. Both the chlorides are soluble in organic solvents and are strong Lewis acids. They are used as Friedel Craft catalysts.
(iv) Beryllium and aluminium ions have strong tendency to form complexes, BeF42–, AlF63–.
Calcium
- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
SOME IMPORTANT COMPOUNDS OF CALCIUM
Quick Lime (solid Calcium Oxide or CaO)
It is prepared on a commercial scale by heating limestone (CaCO3) in a rotary kiln at 1070-1270K.
CaCO3 ⇋ CaO + CO2:
Slaked lime (solid Calcium Hydroxide Ca(OH)2) Calcium hydroxide is prepared by adding water to quick lime, CaO. It is a white amorphous powder. It is sparingly soluble in water.
Lime water is the aqueous solution of Ca(OH)2
Milk of lime is the suspension of slaked lime in water. When carbon dioxide is passed through lime water it turns milky due to the formation of calcium carbonate.
Ca(OH)2 + CO2 → CaCO3 + H2O
On passing excess of carbon dioxide, the precipitate dissolves to form calcium bicarbonate.
CaCO3 + CO2 +H2O→Ca(HCO3)2
CALCIUM CARBONATE CaCO3
Calcium carbonate occurs in nature in several forms like limestone, chalk, marble etc. It can be prepared by passing carbon dioxide through slaked lime or by the addition of sodium carbonate to calcium chloride.
Ca(OH)2 + CO2 → CaCO3 + H2O and CaCl2 + Na2CO3 → CaCO3 + 2NaCl
Calcium carbonate is a white fluffy powder. It is almost insoluble in water. It reacts with dilute acid to liberate carbon dioxide.
Calcium Sulphate (Plaster of Paris), CaSO4·½H2O
It is a hemihydrate of calcium sulphate. It is obtained when gypsum, CaSO4·2H2O, is heated to 393K.
2(CaSO4.2H2O) → 2(CaSO4).H2O + 3H2O
Cement: is a product obtained by combining a material rich in lime, CaO with other material such as clay which contains silica, SiO2 along with the oxides of aluminium, iron and magnesium. The average composition of Portland cement is: CaO, 50-60%; SiO2, 20-25%; Al2O3, 5-10%; MgO, 2-3%; Fe2O3, 1-2% and SO3, 1-2%. For good quality cement, the ratio of silica (SiO2) to alumina (Al2O3) should be between 2.5 and 4 and the ratio of lime (CaO) to the total of the oxides of silicon (SiO2) aluminium (Al2O3) and iron (Fe2O3) should be as close as possible to 2

 Kaysons Publication
Kaysons Publication
