- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
SOLID IN LIQUID SOLUTIONS
Solubility of solid (or Non-volatile liquids) in Liquid
Colligative Properties: The properties of solutions which depends on the no. of solute particles but irrespective of nature of solute particles.
There are four Colligative properties:
a. Relative lowering of vapour pressure
b. Elevation of boiling point
c. Depression of freezing point
d. Osmotic pressure
a. Relative lowering of vapour pressure
P = poA XA + PoB XB → Raoult’s law
Dilute solution is where nA < < nB
Or XA < < < XB
Non volable solute is where ![]()
![]()
![]()
![]()
![]()
b. Elevation of boiling point
Boiling point is the temperature at which the vapour pressure of liquid equals the atmospheric pressure.
Elevation in boiling point (∆Tb) is the difference in boiling point of pure liquid and boiling of solution of this liquid with a non-volatile solute.
![]()
Solutions
As can be clean seen from graph
![]()
![]()
![]()
![]()
![]()
![]()
Kb = Boiling point elevation constant of modal elevation constant (Ebullioscopic) constant
c. Depression of freezing point:
Freezing point: Temperature at which the vapour pressure of the substance in its liquid phase is equal to its vapour pressure in its solid phase
The lowering of vapour pressure of solution causes a lowering of freezing point compared to that of pure solvent. The difference in freezing point of the pure solvent and solution is called depression in freezing point
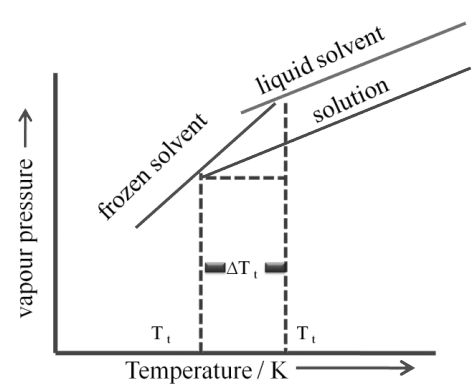
Depression in freezing point
Similar to elevation in boiling point
∆Tf α ∆P
α XA P˚ solvent
α molality
![]()
Kf = depression in freezing point constant or molal depression constant or cryoscopic constant.

 Kaysons Publication
Kaysons Publication
