- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
d. Osmotic pressure
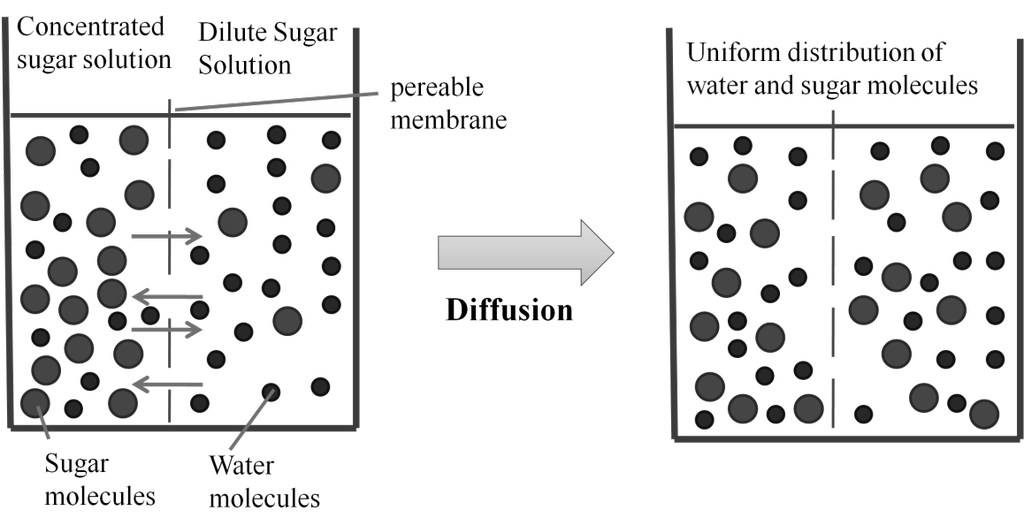
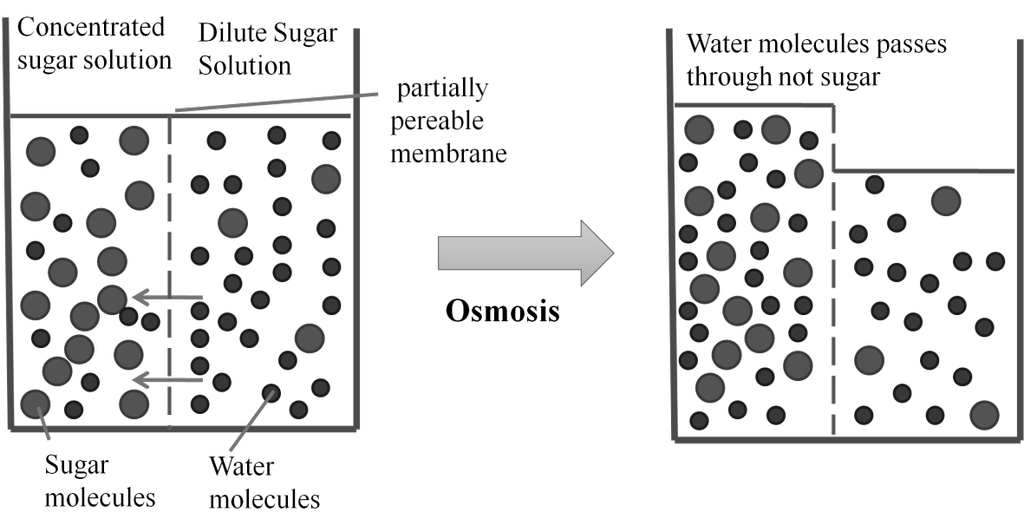
Osmosis: The process of flow of solvent molecules from pure solvent to the solution through semi permeable membrane.
Osmotic pressure (p) : Minimum pressure that must be applied to the solution to prevent the flow of solvent into the solution through a semipermeable membrane
p(Osmotic pressure) = nVRTi=wmVRTi
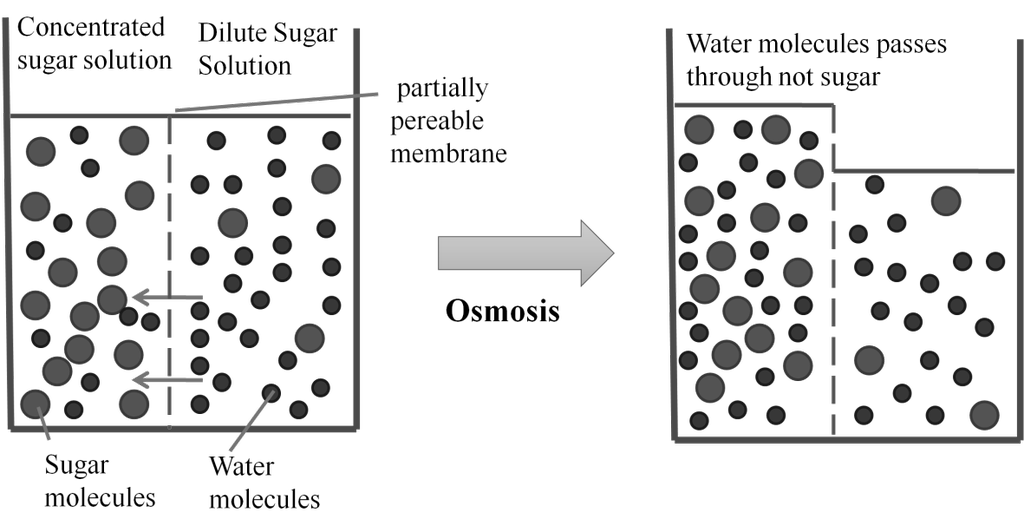
Isotonic solution: Two solutions having same osmotic pressure at a given temperature are called isotonic solution p1 = p2
Hypertonic solutions: If a solution has more osmotic pressure than other solution it is called hypertonic solution.
Hypotonic solution: If a solution has less osmotic pressure than other solution it is called hypotonic solution
Reverse osmosis: When a pressure larger than osmotic pressure is applied to the solution side, the pure solvent flows out of solution to solvent side through semi permeable membrane this process is called reverse osmosis Use: Reverse osmosis is used to desalination of sea water (into drinking water)
Application of Colligative property: By measuring a colligative property of a solution molar mass of the non-volatile solute can be calculated Conditions for getting accurate value of molar mass:
- Solute must be non-volatile
- Solution must be dilute
- Solute particles must not undergo any association or dissociation in the solution
Abnormal molar mass and Van’t Hoff factor (i): When the non-volatile solute particles undergo association (Eg: acetic acid in benzene) or dissociation (Eg: electrolytes like KCl, NaCl in water), the abnormal molar mass is observed.
NaCl No+ + Cl-
1 mole 0 0 total moles = 0
1- α α α total moles= 1+α
α = degree of dissociation
Actual moles are (1 + α) not (1)
![]()
![]()
![]()
1. For solutions undergoing dissociation
i >1 { Exp coll .prop > normal cell. Property}
{exp. Mol. wt < normal mol.wt }
2. For solution undergoing association
i < 1 { exp. Coll. Property < normal. Coll. property}
{ exp. Mol. Wt > normal .mol .wt}
![]()
![]()
Association of solute
CH3COOH Anociation in benzene to form dimer
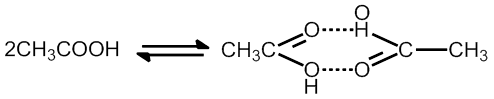
Note CH3COOh dissociate in water
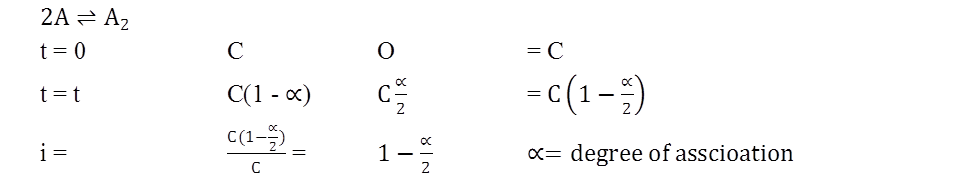
General formula (both for anociation an well an dissociation)
![]()
y = no. of particle from parhole.
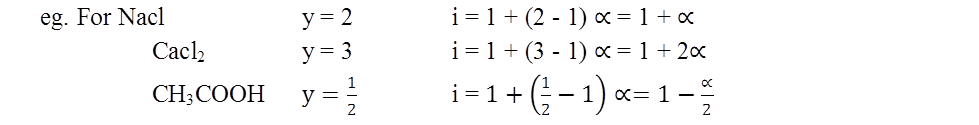
Important Formulae:
- p(osmotic pressure) =
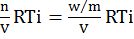
- Isotonic solution p1 = p2
- Vapour pressure variation with temperature 2.303 log10
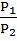 =
= 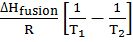
- Raoult’s Law For liquid-liquid systems: P=PPA+PPB+…
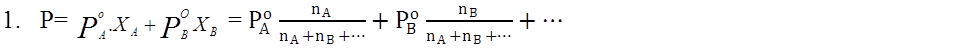
Relative lowering of vapour pressure:
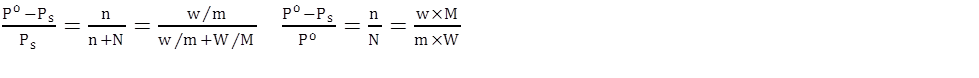 [for dilute solutions]
[for dilute solutions]
- Elevation in boiling point. ∆Tb=Kb×molality xi=1000 KbwmWi
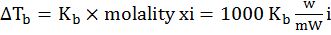
- Depression in freezing point: ∆Tf=Kf×molality xi=1000 KfwmWi
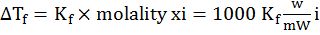
 and
and 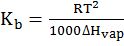
- For solutes undergoing dissolution AxByà xAy++ yBx- i>1. Experimental colligative property > normal colligative property and Experimental molecular weight < normal molecular weight
- For solutes undergoing association A +A +..nà An i<1 Experimental colligative property < normal colligative property and Experimental molecular weight > normal molecular weight
- Vant Hoff Coefficient i = 1 + (y-1)a a=degree of dissociation or association and y = no of particles from one particle

![]()

 Kaysons Publication
Kaysons Publication
