- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
INTRODUCTION
The word .atom has been derived from the Greek word atomio which means .un-cutable or non-divisible.
DALTON’S ATOMIC THEORY
The matter is composed of small indivisible particles called atoms (from Greek word atomio, meaning indivisible). In 1808 Dalton proposed the following theory
- Matter consists of atoms, which cannot be divided further.
2. All the atoms of a given element have identical mass. Atoms of different elements differ in mass.
3. Atoms combine in a fixed ratio to form Compounds.
4 The atoms cannot be created or destroyed.
SUCCESS OF DALTON’S ATOMIC THEORY
- Atoms are the smallest part of matter
- Atoms cannot be created of destroyed
- Atoms of same element are similar in mass
- Atoms combine with each other in simple ratios.
FAILURES OF DALTON’S ATOMIC THEORY
It could explain lows of chemical combination, as on today we know that all four points are not correct.
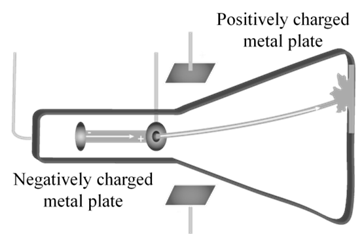
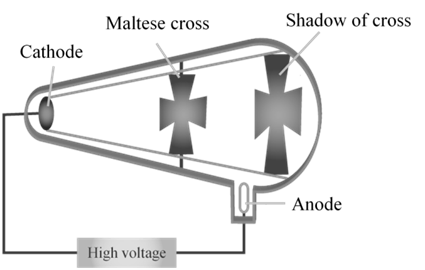
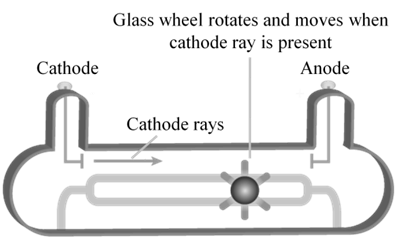
CATHODE RAYS
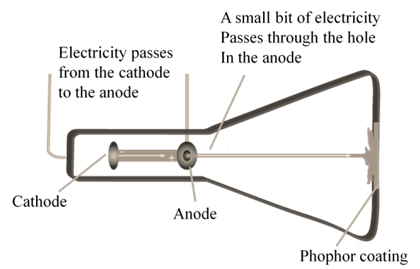
CATHODE RAYS ARE NEGATIVELY CHARGED
CATHODE RAYS TRAVEL IN STRAIGHT LINE.
CATHODE RAYS ARE PARTICLES
CHARGE /MASS (E/M) RATIO
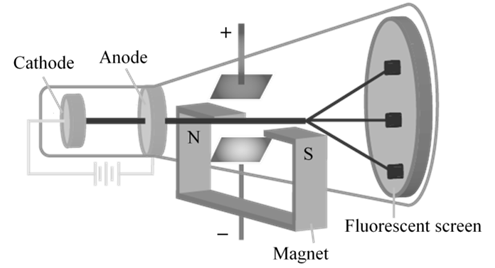
In 1897 JJ. Thomson determined the e/m value of cathode rays.
Thomson proved that e/m ratio is same, whatever material is of plates or gas filled in the tube
![]()
Ex.: Which of the following is never true for cathode rays?
- They possess kinetic energy
- They are electromagnetic waves
- They produce heat
- They produce mechanical pressure Answer (c)
MILLIKAN’S OIL DROP EXPERIMENT
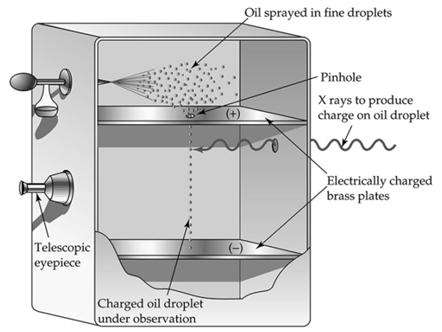
MASS OF ELECTRON
From Millikan's oil drop experiment e = 1.6022 x 10-19 coulombs
![]()
![]()
![]()
ANODE RAYS
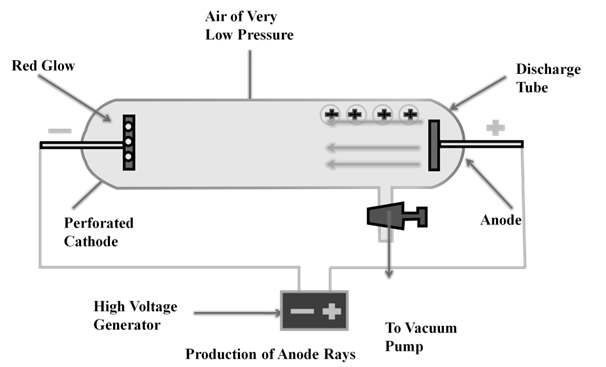

The smallest and lightest positive ion was obtained from hydrogen and was called proton. This characterized in 1919.
Later the presence of electrically neutral particle was found in the atom. These particles were discovered by Chadwick (1932) by bombarding a thin sheet of beryllium by α-particles, then electrically neutral particles having a mass slightly greater than that of the protons was emitted. He named these particles as neutrons.
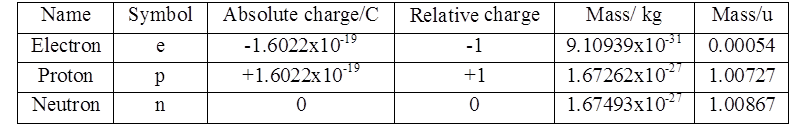
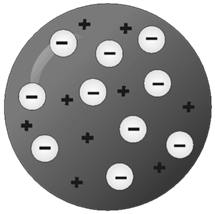
Thomson Model of Atom
Plum pudding model OR
Water melon model
FUN FACTS JJ THOMSON (NOBEL PRIZE IN 1906)


 Kaysons Publication
Kaysons Publication
