- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
CRYSTAL LATTICES
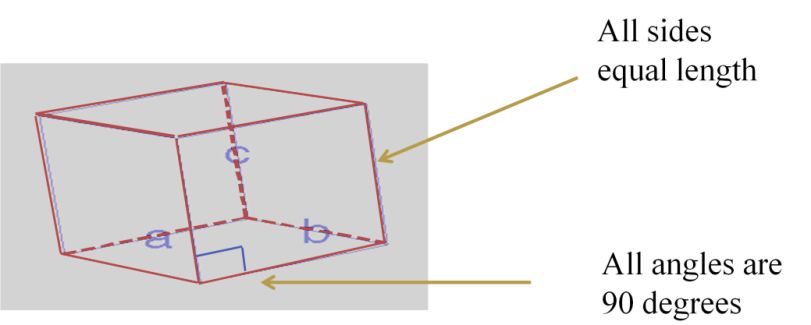
- Lattice: Regular 3-D arrangements of equivalent LATTICE POINTS in space.
- UNIT CELLS
- Smallest repeating internal unit that has the symmetry characteristic of the solid.
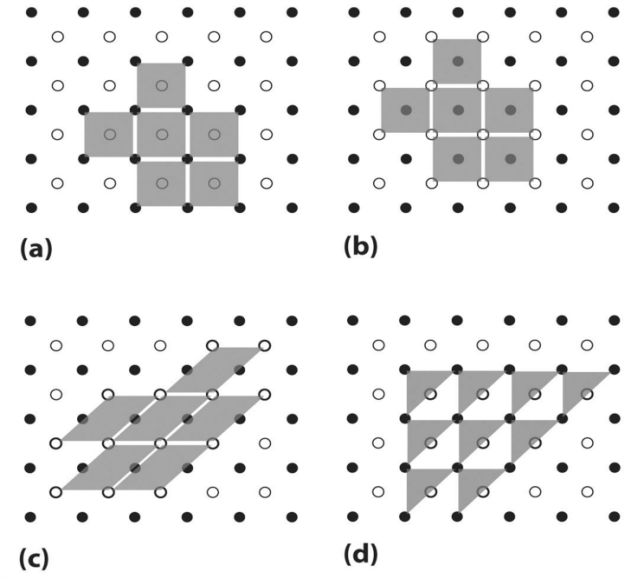
- Actual unit cell
- Unit cell as drawn
- Whole crystal
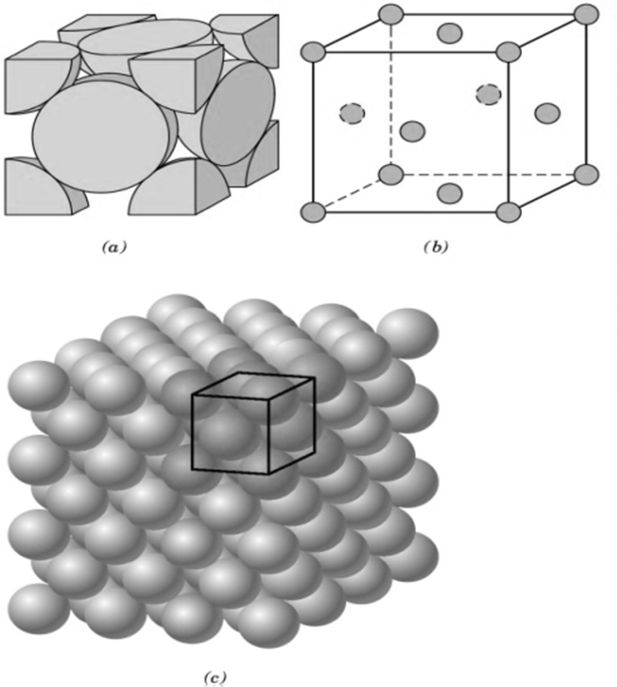
CRYSTAL LATTICE GEOMETRY
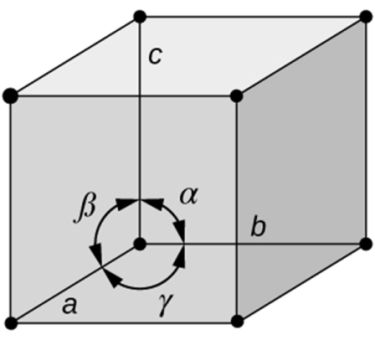
- Based on shape of unit cell ignoring actual atomic locations
- Unit cell = 3-dimensional unit that repeats in space
- Unit cell geometry completely specified by a, b, c (distance in x, y & z axis)& a, b, g (angles between a,b & c (lattice parameters or lattice constants)
- Seven possible combinations of a, b, c & a, b, g, resulting in seven basic crystal systems
14 Bravais Lattices: seven basic lattices and fourteen overall
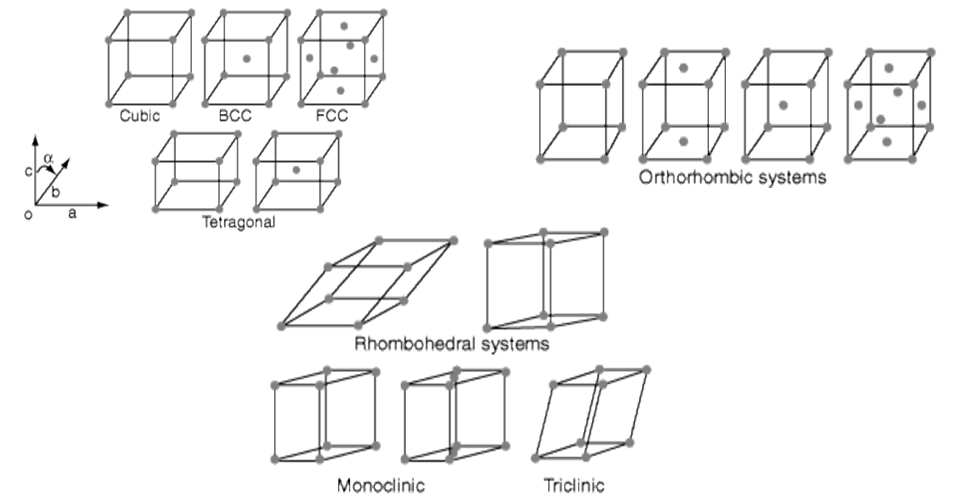
14 Bravais Lattices from seven basic lattices
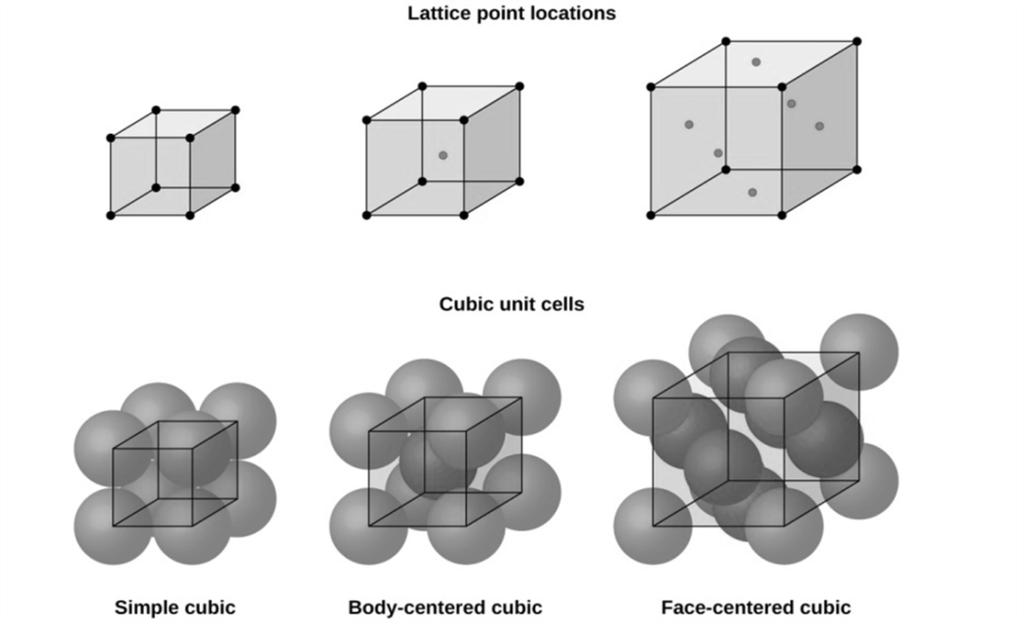
Cubic Unit Cells
There are 7 basic crystal systems, but we are only concerned with CUBIC.
DIFFERENT CUBIC UNIT CELLS
ATOM SHARING AT CUBE FACES AND CORNERS
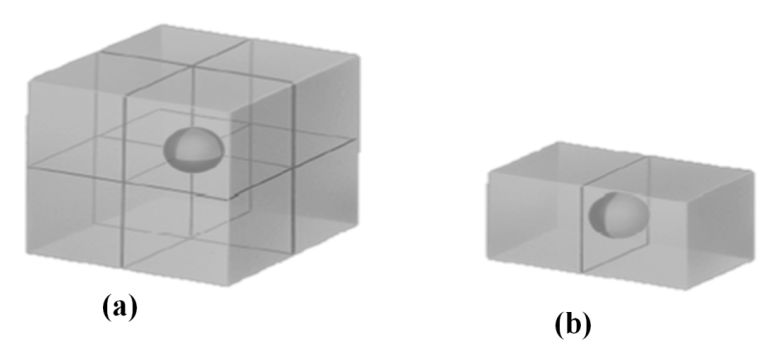
Atom shared in corner
à 1/8 inside each unit cell
Atom shared in face
à ½ inside each unit cell
Atoms shared on edges
à ¼ inside each unit cell
Atoms inside the unit cell
à 1 inside each unit cell
NUMBER OF ATOMS PER UNIT CELL
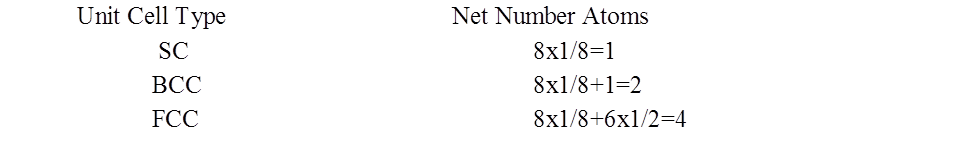
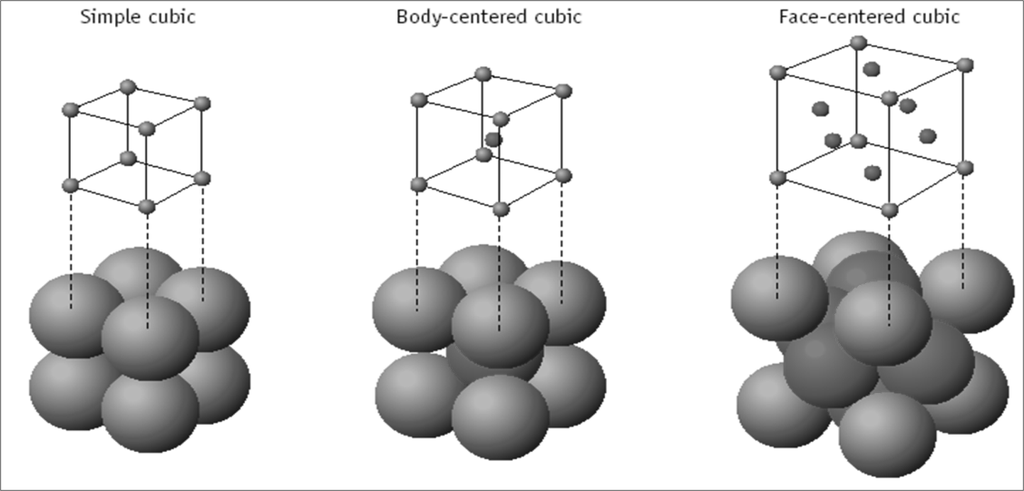
PACKING FRACTION
![]()
![]()
![]()
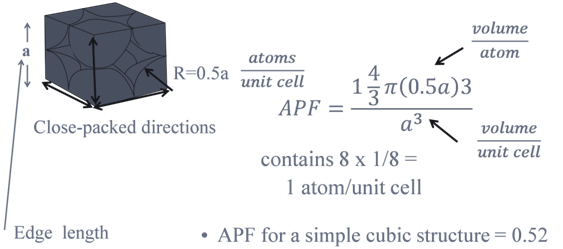
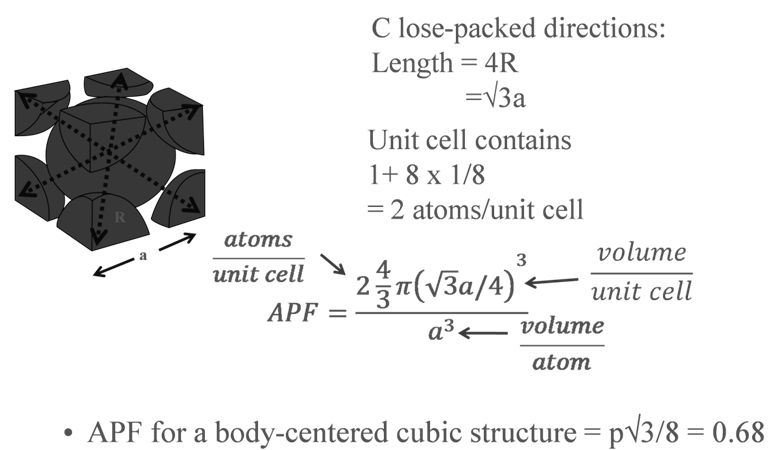
a = edge length of unit cell
R = radius of atoms( assuming they are hard spheres)
Z= no of atoms in one unit cell
PACKING FRACTION: SC
PACKING RACTION: BCC
PACKING FRACTION: FCC

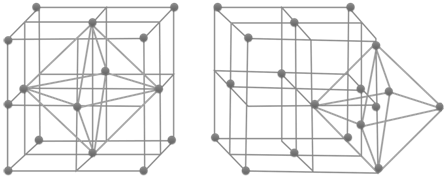
Voids:- gaps between atoms in a crystal are called voids.
- There are two types of voids
- Tetrahedral voids and octahedral voids
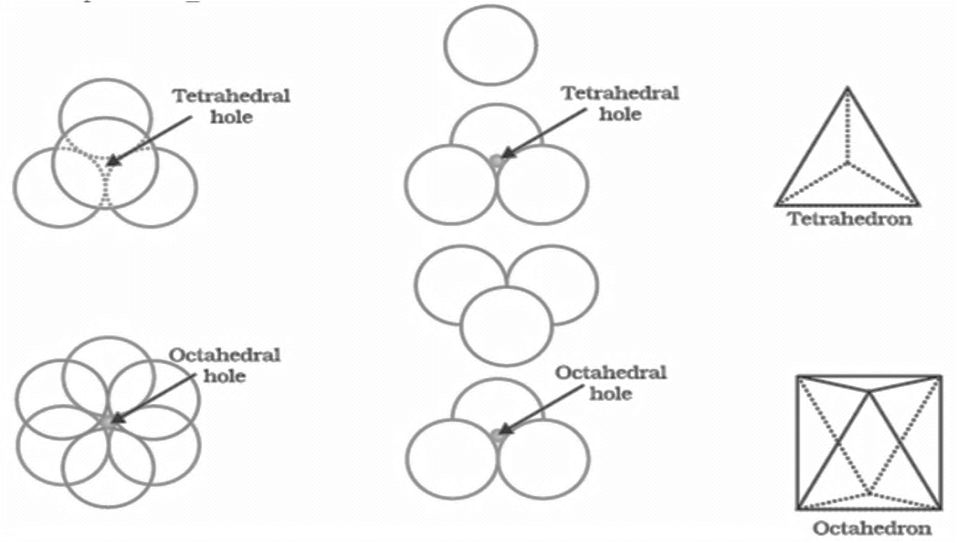
HOW TO LOCATE VOIDS
- No. of tetrahedral voids = 2 x effective atoms/unit cell
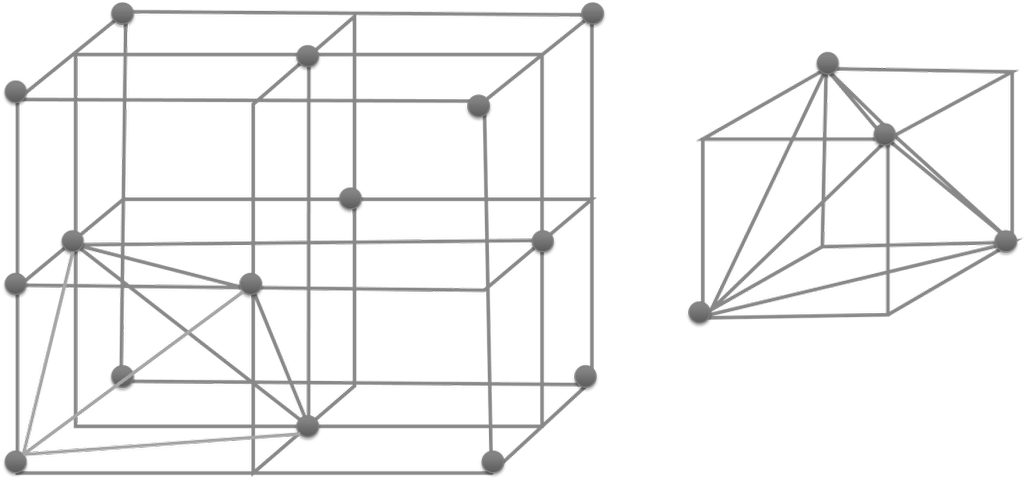
- No. of octahedral voids = 1 x effective atoms/unit cell
COMPARISON OF CRYSTAL STRUCTURES
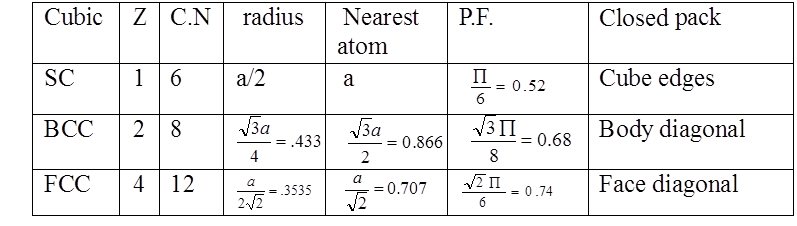

 Kaysons Publication
Kaysons Publication
