- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Every field of science involves taking measurements, understanding them, and communicating them to others. In other words, we all have to speak the same basic language. Whether you are a chemist, a physicist, a biologist, an engineer, or even a medical doctor, you need a consistent way of communicating size, mass, shape, temperature, time, amount, energy, power, and speed.
The International System of Units (abbreviated SI, from the French Système international d’unités) is the metric system used in science, industry, and medicine. Depending on your age and geographic location, you might be very familiar with the “imperial” system, which includes units such as gallons, feet, miles, and pounds. The imperial system is used for “everyday” measurements in a few places, such as the United States. But in most of the world (including Europe) and in all scientific circles, the SI system is in common use.
The SI is made up of 7 base units that define the 22 derived units with special names and symbols.
History of the SI System

The SI units of measurement have an interesting history. Over time they have been refined for clarity and simplicity.
- The meter (m), or metre, was originally defined as 1/10,000,000 of the distance from the Earth’s equator to the North Pole measured on the circumference through Paris. In modern terms, it is defined as the distance traveled by light in a vacuum over a time interval of 1/299,792,458 of a second.
- The kilogram (kg) was originally defined as the mass of a liter (i.e., of one thousandth of a cubic meter). It is currently defined as the mass of a platinum-iridium kilogram sample maintained by the Bureau International des Poids et Mesures in Sevres, France.
- The second (s) was originally based on a “standard day” of 24 hours, with each hour divided in 60 minutes and each minute divided in 60 seconds. However, we now know that a complete rotation of the Earth actually takes 23 hours, 56 minutes, and 4.1 seconds. Therefore, a second is now defined as the duration of 9,192,631,770 periods of the radiation corresponding to the transition between the two hyperfine levels of the ground state of the cesium-133 atom.
- The ampere (A) is a measure of the amount of electric charge passing a point in an electric circuit per unit time. 6.241×1018 electrons, or one coulomb, per second constitutes one ampere.
- The kelvin (K) is the unit of the thermodynamic temperature scale. This scale starts at 0 K. The incremental size of the kelvin is the same as that of the degree on the Celsius (also called centigrade) scale. The kelvin is the fraction 1/273.16 of the thermodynamic temperature of the triple point of water (exactly 0.01 °C, or 32.018 °F).
- The mole (mol) is a number that relates molecular or atomic mass to a constant number of particles. It is defined as the amount of a substance that contains as many elementary entities as there are atoms in 0.012 kg of carbon-12.
- The candela (cd) was so named to refer to “candlepower” back in the days when candles were the most common source of illumination (because so many people used candles, their properties were standardized). Now, with the prevalence of incandescent and fluorescent light sources, the candela is defined as the luminous intensity in a given direction of a source that emits monochromatic radiation of frequency 540⋅1012
Derived units – are defined in terms of the seven base quantities via a system of quantity equations. The SI derived units for these derived quantities are obtained from these equations and the seven SI base units
For ease of understanding and convenience, 22 SI derived units have been given special names and symbols,
Ex: volume = L x L x L = m3 or cm3
Density=massvolume=kgm3 or gmm3
Force: unit N (Newton) = kg.m/sec2
Conversion of one unit to another example
m3 = m x m x m = (100cm) x (100) cm x (100) cm = 106 cm3
Density kgm3=1000gm106cm3=10-3 gmcm3
Measurement of data: when conducting experiments we have to measure & report data. There are certain norms for reporting this data.
All very big and small values are expressed in exponents
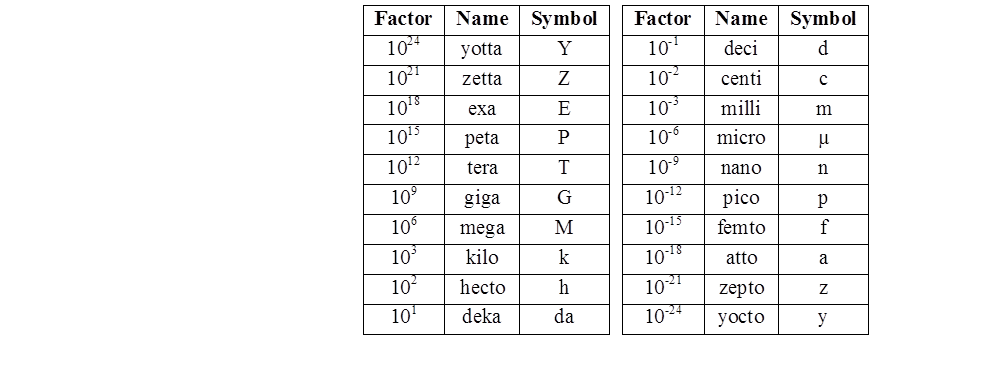
PRECISION AND ACCURACY
- Precision indicates how closely repeated measurements match each other.
- Accuracy indicates how closely a measurement matches the correct or expected value.
- A result is valid only if it is both accurate and precise.
EXAMPLE
- If the true value for a result is 8.00 kg and a student “A” takes two measurements and reports the results as 7.95 kg and 7.93 kg. These values are precise as they are close to each other but are not accurate.
- Another student “B” repeats the experiment and obtains 7.94 kg and 8.05 kg as the results for two measurements. These observations are neither precise nor accurate.
- When a third student “C” repeats these measurements and reports 8.01 kg and 7.99 kg. These values are both precise and accurate.
SIGNIFICANT FIGURES
Significant figures are meaningful digits which are known with certainty. The uncertainty is indicated by writing the certain digits and the last uncertain digit. Thus, if we write a result as 11.2 ml, we say the 11 is certain and 2 is uncertain and the uncertainty would be ±1 in the last digit.
Unless otherwise stated, an uncertainty of ± 1 in the last digit is always understood.
RULES FOR DETERMINING THE NUMBER OF SIGNIFICANT FIGURES
- All non-zero digits are significant
- Zeros preceding the first non-zero digit are not significant
- Zeros between two non-zero digits are significant.
- Zeros at the end or right of the number are significant provided they are on the right side of the decimal point. But, if otherwise, the zeros are not significant
- Counting numbers of objects, for example, 2 balls or 20 eggs, have infinite significant figures as these are exact numbers
EXAMPLE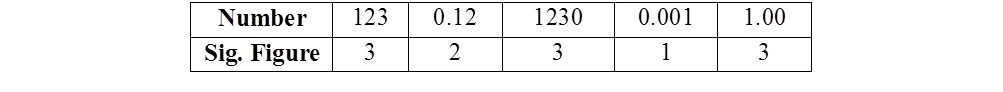
ADDITION AND SUBTRACTION OF SIGNIFICANT FIGURES
During addition and subtraction, the result cannot have more digits to the right of the decimal point than either of the original numbers.
MULTIPLICATION AND DIVISION OF SIGNIFICANT FIGURES
In multiplication and division with significant figures, the answer cannot have more significant figures than either of the original numbers.
Example Example
2.12 1.2
+ 6.1 x 1.3
0.012 1.56
8.232 Ans. 1.6
Ans.= 8.2
ROUNDING OFF THE SIGNIFICANT FIGURES
1. If the rightmost digit to be removed is more than 5, the preceding number is increased by one. For example, in figure 2.486 if we have to remove 6, we have to round it to 2.49
2. If the rightmost digit to be removed is less than 5, the preceding number is not changed.
For example, in figure 6.664 if 4 is to be removed, then the result is rounded of to 6.66.
3. If the rightmost digit to be removed is 5, then the preceding number is not changed, if it is an even number but it is increased by one if it is an odd number. For example, if 2.35 is to be rounded by removing 5, we have to increase 3 to 4 giving 2.4 as the result. However, if 2.25 is to be rounded off it is rounded off to 2.2
Ex.: A student performs a titration with different burettes and finds titre values of 25.2 mL, 25.25 mL, and 25.0 ml. The number of significant figures in the average titre value is
(IIT adv. 2010, integer type)
Sol. During addition and subtraction, the result cannot have more digits to the right of the decimal point than either of the original numbers Answer: 3
Ex.: If the value of Avogadro number is 6.023 × 1023 mol–1 and the value of Boltzmann constant is 1.380 × 10–23 J K–1, then the number of significant digits in the calculated value of the universal gas constant is (IIT adv. 2014, integer type)
Sol. K = R/NA R=KNA
R= 6.023 × 1023 × 1.380 × 10–23 J.mol–1.k–1= 8.31174 J.mol–1.k–1. There are 4 significant figures in each term. (4) Hence, these be 4 significant figure in R Answer. 4

 Kaysons Publication
Kaysons Publication
