- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
SHIELDING EFFECT
Shielding effect or screening effect: Due to the presence of electrons in the inner shells, the electron in the outer shell will not experience the full positive charge on the nucleus.
So due to the screening effect, the net positive charge experienced by the electron from the nucleus is lowered and is known as effective nuclear charge.
- Effective nuclear charge, Zeff, experienced by an electron is less than actual nuclear charge , Z
- Electrons in the outermost shell are repelled (shielded) by electrons in the inner shells. This repulsion counteracts the attraction caused by the positive nuclear charge
Coulomb’s law:

SHIELDING EFFECT
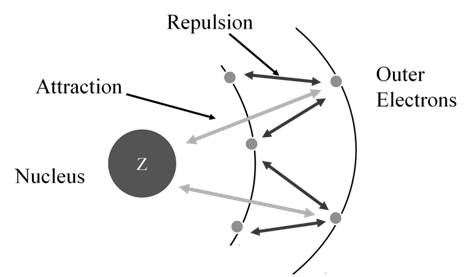
Effective nuclear charge, Zeff, experienced by an electron is less than the actual nuclear charge, Z. „Electrons in the outermost shell are repelled (shielded) by electrons in the inner shells. This repulsion counteracts the attraction caused by the positive nuclear charge
Zeff = Z – S (S = screening constant)
Shielding effect
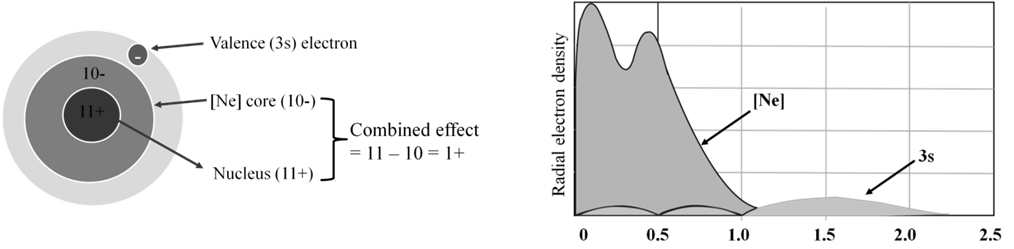
- Electrons in inner orbitals have greater shielding effect than electrons in same shell.
- Shielding effect s > p > d > f
ATOMIC RADII
Periodicity
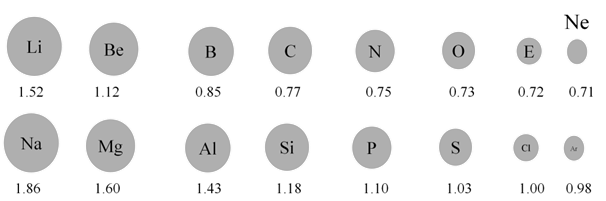
- As we move from left to right along the period, the effective nuclear charge "felt" by the outermost electron increases while the distance from the nucleus doesn't change that much (electrons are filling the same shell)
- Outermost electrons are attracted stronger by the nucleus, and the atomic radius decreases

IONIC RADII
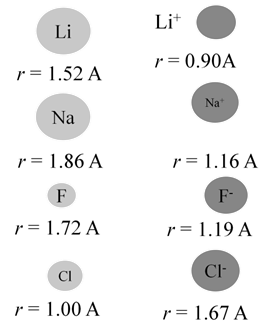
When atom loses an electron, its radius always decreases
- Cations (positive ions) are always smaller than their respective neutral atoms.
When atom gains an electron, its radius always increases
- Anions (negative ions) are always larger than their respective neutral atoms
ISOELECTRONIC SPECIES
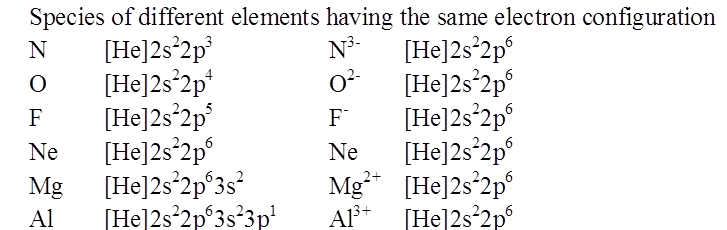
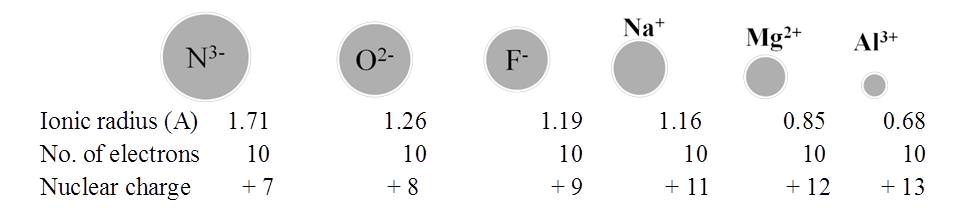
RADII OF ISOELECTRONIC IONS

 Kaysons Publication
Kaysons Publication
