- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Molecules
The smallest particle of a substance which is capable of independent existence is called a molecule. In general, molecule is a group of two or more atoms that are chemically bonded together. It shows all the properties of the substance. Molecules can be divided into two categories.
Molecules of Elements
The molecules of an element are constituted by the same type of atoms. Molecules of many elements are made up of one atom of that element. e.g. noble gases like argon (Ar), helium (He), etc.
The molecules of most of more than one atoms of the non-metals are made up of more than one atoms.
For Example:- A molecule of oxygen consists of two atoms of oxygen. Ozone (O3) consists of three atoms of oxygen.
Atomicity
It is defined as the number of atoms present in a molecule.
On the basis of atomicity, molecules can be classified as
(i):- Monatomic Molecules They consist of only one atom. e.g. He, Ne, Ar, Xe, Fe, Al, etc.
(ii):- Diatomic Molecules They consist of two atoms. e.g. H2,O2, N2,I2, Br2, C12, etc.
(iii):- Triatomic Molecules They consist of three atoms. e.g. O3.
(iv):- Tetraatomic Molecules They consist of four atoms. e.g. P4.
(v):- Polyatomic Molecules They consist of more than four atoms. e.g. S8.
Molecules of Compounds
Atoms of different elements join together in definite proportions forming molecules of compounds.
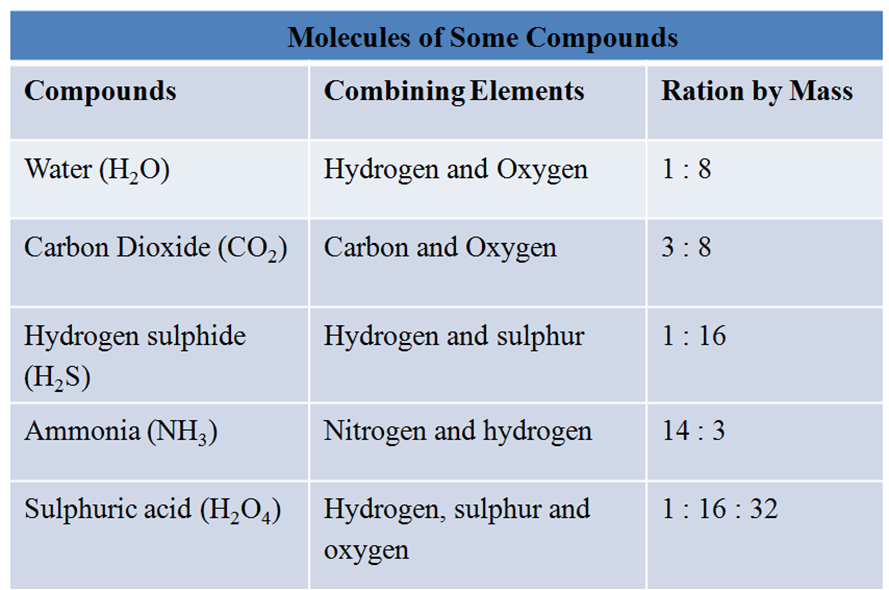
For Example, H2O molecule is made up of hydrogen and oxygen elements in the ratio of 1:8 by mass.
Ions
When atoms, groups of atoms or molecules lose or gain electron(s) they become charged. These charged species are known as ions. These can be negatively or positively charged.
Cations
The positively charged ions which are attracted towards cathode in an electric field are known as cations. e.g. Na+, K+, Ca2+, A13+ , etc.
Anions
The negatively charged ions which are attracted towards anode in an electric field are known as anions. e.g. Cl–, Br–, O, N3-, etc.
Ionic Compounds
The compound which consists of ions as its constituent particles is known as ionic compound. It contains ionic bonds. e.g. sodium chloride, consists of a positively charged) sodium ion (Na+ cation) and negatively charged chloride ion (C1- anion). Calcium oxide or quicklime (CaO) consist of calcium cation (Ca2+) and oxide anion (O2-).
Polyatomic Ion
The compound which consists of ions as its constituent particles is known as ionic compound. It contains ionic bonds. e.g. sodium chloride, consists of a positively charged) sodium ion (Na+ cation) and negatively charged chloride ion (C1- anion). Calcium oxide or quicklime (CaO) consist of calcium cation (Ca2+) and oxide anion (O2-).
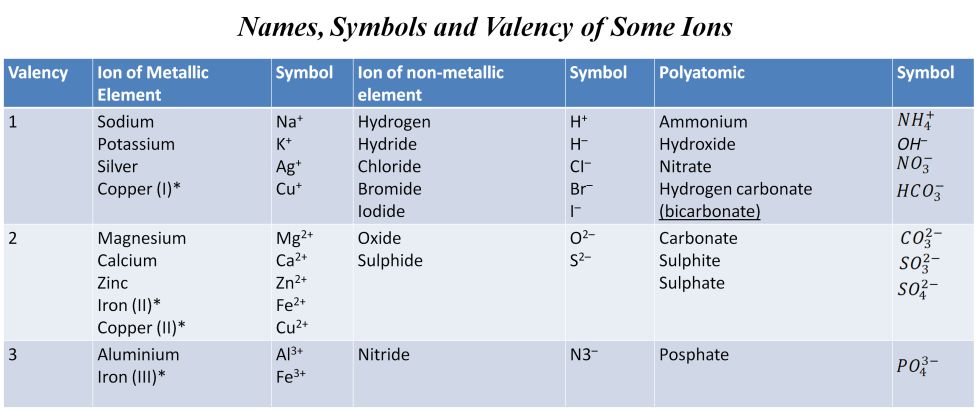
Valency
The combining power (or capacity) of an element is called its valency. Valency can be used to find out how the atoms of an element will combine with the atom(s) of another element to form a chemical compound.

 Kaysons Publication
Kaysons Publication
