- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Chapter:- 3
Atoms and Molecules
Introduction
We shall discuss about the various laws which indicates how atoms combine to form molecule, symbols and formula of atoms and compounds and various ways of expressing their masses.
Laws of Chemical Combination
Whenever reactants react together to form the products or the elements combine together to form a compound, they do this according to certain laws. These laws are called the laws of chemical combination.
Law of Conservation of Mass
It states, "Mass can neither be created nor be destroyed in a chemical reaction."
Law of Constant Proportions/ Law of Definite Proportions
French chemist, joseph Proust analysed the chemical composition of a large number of compounds and came to the conclusion that the proportion of each element in a compound I constant. On this basis he proposed the law of constant proportions.
"A pure chemical compound always consists of the same elements that are combined together in a fixed (or definite) proportion by mass."
For Example:- carbon dioxide (CO2) always contains carbon and oxygen in the ratio of 3 : 8. If a sample of CO , contains 36 g of carbon then it is compulsory that it has 96g Oxygen.
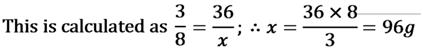

 Kaysons Publication
Kaysons Publication
