- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Solution containing volatile solute / solvents
Mixture of 2 volatile liquids
Raoult's law ( for volatile Liq. Mixture )
Statement of Raoult's law ( for volatile liq. mixture ): In solution of volatile liquids, the partial vapour pressure of each component is directly proportional to its mole fraction.
pA µ xA => pA = xAPAº
pA = Partial vapour pressure of component A
xA = Mole fraction of component ‘A’ in solution.
PAº = Vapour pressure of pure component ‘A’ at given temperature
Derivation of total pressure over solution using Raoult’s law and Dalton’s law:
Let A, B be to two volatite liquids in a closed container as shown.
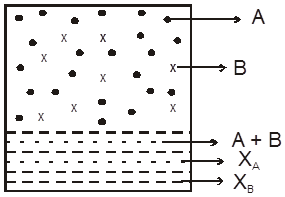
pA = xAPAº
Similarly, for liquid B we have,
pB = xBPBº
Total pressure over the solution PT , according to Dalton's law is
PT = pA + pB = xAPA0 + xBPB0
Determining composition of vapour phase:
Let,
yA = mole fraction of A in vapour phase above the solution and
yB = mole fraction of B in vapour phase above the solution
Now, we have, pA = yA PT .....Dalton's law of partial pressure for a gaseous mixture
pA = xAPAº ...........Raoult's law
Thus, pA = yA PT = xA PAº
Also, pB = yBPT = xBPBº
xA + xB = 1 = 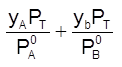 ; Thus
; Thus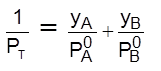
Graphical Representation of Raoult's Law:
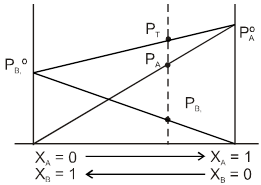
pA= xAPAº & pB = xB PBº
PT = xAPAº + xB PBº
PT = ( PAº – PBº ) xA + PB0
PT = ( PBº – PAº ) xB + PA0
This represents equation of straight line. PT v.s. x
Note: If PAº > PBº , A is more volatile than B. B.P. of A < B.P. of B.
Limitations of Raoult’s Law: Raoult's Law only works for ideal solutions. Very dilute solutions obey Raoult's Law to a reasonable approximation.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
