Chapter 9
Biomolecules
The biosphere has a vast variety of living species. Carbon, hydrogen, oxygen, and numerous other elements, as well as their respective contents, are acquired per unit mass of living tissue when an elemental analysis is done on plant tissue, animal tissue, or microbial paste. A similar list of elements will be obtained if the same analysis is performed on a piece of the earth's crust as an example of non-living stuff. A sample of live tissue contains all of the elements found in a sample of the earth's crust. However, a closer look reveals that any living organism has a higher relative quantity of carbon and hydrogen in relation to other elements than the earth's crust.
How to Analyse Chemical Composition?
A natural substance's elemental analysis reveals that it is made up of several elements such as carbon, hydrogen, oxygen, chlorine, and so on. Analytical procedures provide information on various organic and inorganic compounds, as well as their molecular formulas and structures. They also aid in the separation and purification of one component from another. Simple experiments can be used to determine the chemical composition of biomolecules. Crush and combine a piece of living tissue with an acid. We get two pieces after filtering it. The acid-soluble fraction of the filtrate is retained on the filter membrane, while the acid-insoluble fraction is retained on the filter membrane. This indicates that there are two or more substances with distinct characteristics within the tissues.Thousands of organic molecules have been discovered in the acid-soluble pool by scientists.
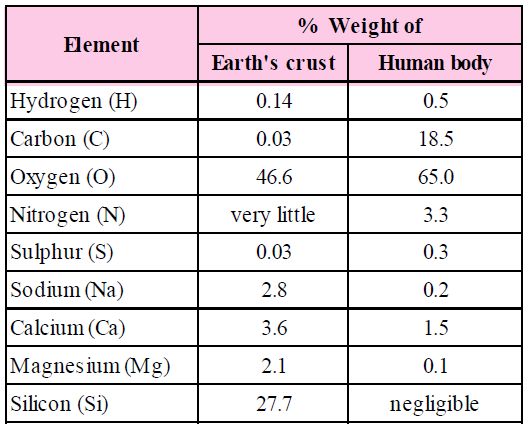
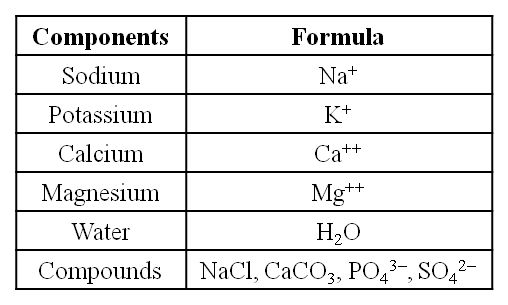
Constituents of Living Tissues
Take another piece and burn it till all the moisture in it is evaporated. When carbon compounds are burned, they are all oxidized. Inorganic substances such as calcium, magnesium, sulfate, phosphate, and others are formed in the tissue by the ash that has been left out.
Analytical techniques, when applied to a chemical, provide us with an estimate of its molecular formula and likely structure. 'Biomolecules' refers to all carbon compounds obtained from living tissues. All carbon-containing chemicals (organic compounds) found in living organisms are classified as biomolecules. They are organic substances found in living cells that have a role in the organism's maintenance and metabolic activities. Inorganic elements and compounds, on the other hand, are found in living beings. As a result, elemental analysis provides information on the composition of live tissues in terms of hydrogen, oxygen, chlorine, carbon, and other elements, whereas compound analysis provides information on the types of organic and inorganic constituents found in living tissues.
Functional groups such as aldehydes, ketones, aromatic molecules, and others can be detected in living tissues from a chemical standpoint. However, amino acids, nucleotide bases, fatty acids, and carbohydrates make up living tissues from a biological standpoint.
Amino acids are chemical molecules that have both an amino and an acidic group as substituents on the same carbon, the -carbon. As a result, they are known as -amino acids. They're methanes that have been replaced. The four valency locations are occupied by four substituent groups. Hydrogen, carboxyl group, amino group, and a variable group known as R group are the four groups. There are numerous amino acids in the R group due to its nature. However, there are only twenty varieties of those found in proteins. The R group in these proteinaceous amino acids could be a hydrogen (glycine), a methyl group (alanine), hydroxy methyl (serine), or something else entirely. The amino, carboxyl, and R functional groups make up the chemical and physical properties of amino acids.Acidic (e.g., glutamic acid), basic (lysine), and neutral (valine) amino acids are classified by the number of amino and carboxyl groups they contain.
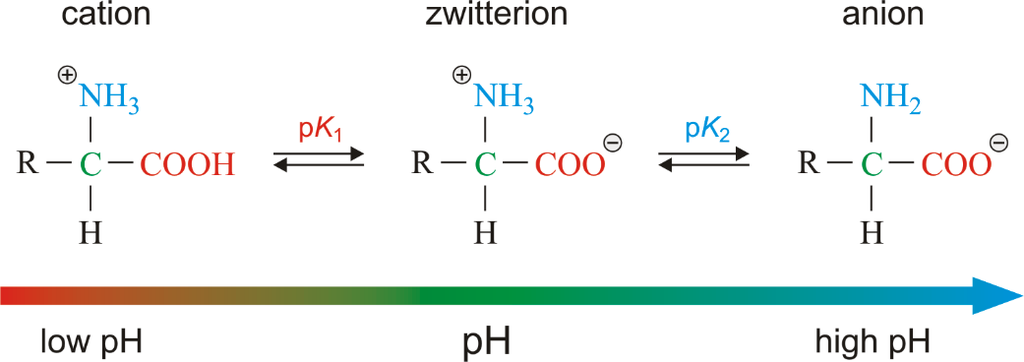
Similarly, aromatic amino acids exist (tyrosine, phenylalanine, tryptophan). The ionizability of the –NH2 and –COOH groups of amino acids is a unique feature. As a result, the structure of amino acids alters in different pH solutions.
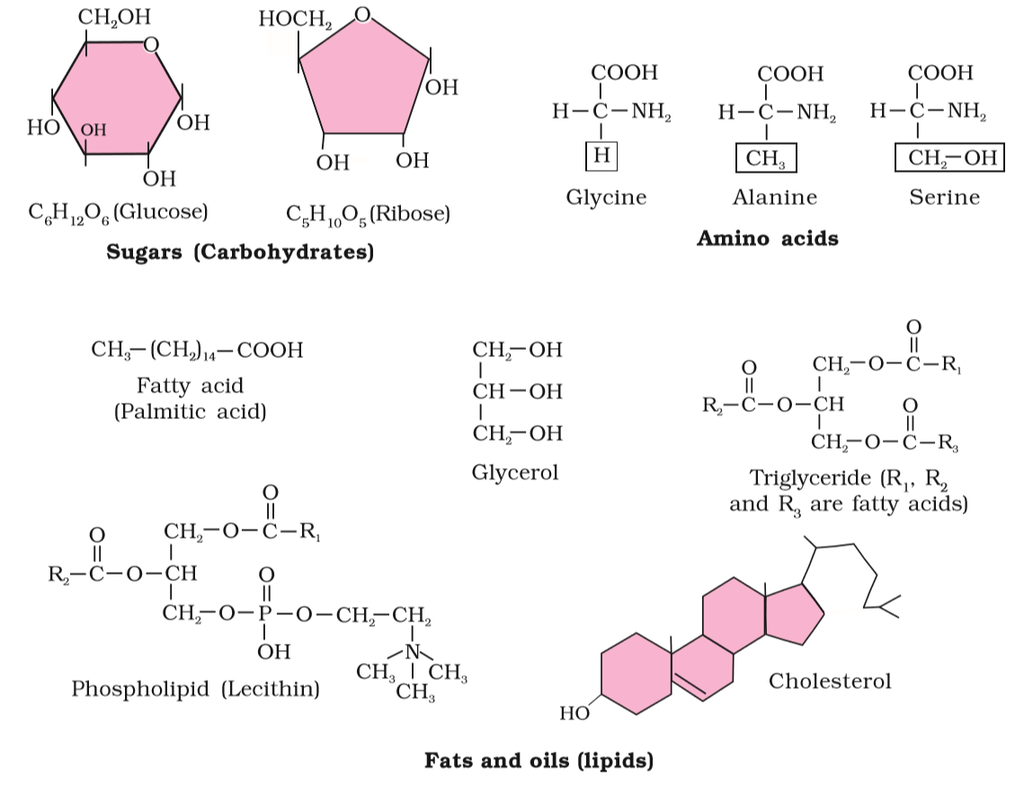
Figure 2 (a): Diagrammatic representation of small molecular weight organic compounds in living tissues
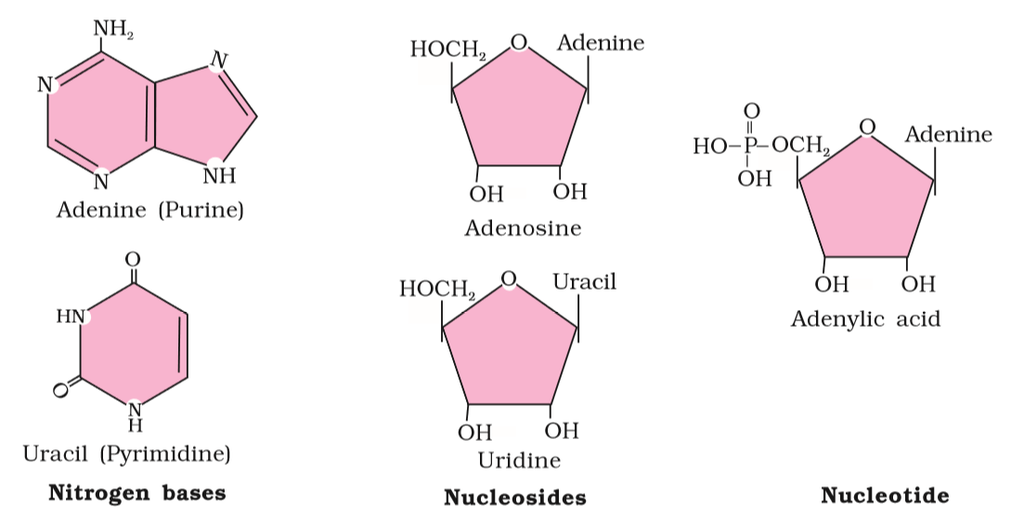
Figure 2(b): Diagrammatic representation of small molecular weight organic compounds in living tissues
Heterocyclic rings can be found in a variety of carbon compounds found in living beings. Adenine, guanine, cytosine, uracil, and thymine are examples of nitrogen bases. They're called nucleosides when they're found linked to sugar. Nucleotides are formed when a phosphate group is additionally esterified to the sugar. Nucleosides include adenosine, guanosine, thymidine, uridine, and cytidine. Nucleotides include adenylic acid, thymidylic acid, guanylic acid, uridylic acid, and cytidylic acid. Only nucleotides make up nucleic acids like DNA and RNA. DNA and RNA are two types of genetic material.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
