- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
LIPIDS
They are made of carbon, hydrogen and little oxygen.
The number of oxygen atoms in a lipid molecule is always less as compared to the number of carbon atoms.
Sometimes small amounts of phosphorus, nitrogen and sulphur are also present.
Lipids are insoluble in water, but soluble in non-polar solvents like chloroform and benzene. lipids contain fatty acids which may be saturated or unsaturated.
Fatty acids are organic acids with a hydrocarbon chain ending in a carboxyl group (COOH).
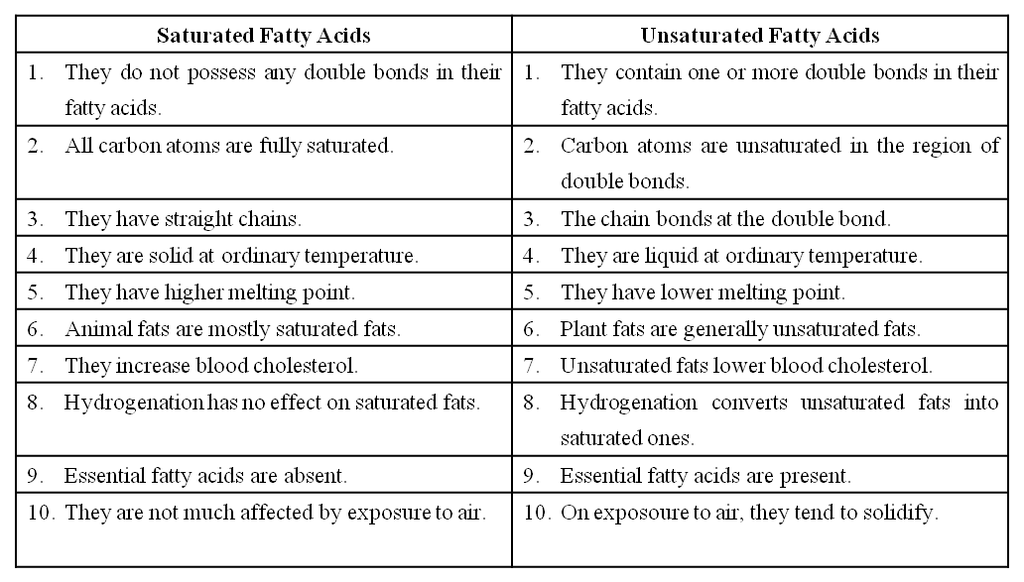
Fatty acids are called saturated if they do not have any double bonds between the carbons of the molecular chain e.g., palmitic acid (16 C) and stearic acid (18 C).
Their melting point is high.
CH3(CH2)14COOH CH3(CH2)16COOH General formula of saturated fatty acids
Palmitic acid Stearic acid CnH2nO2
Unsaturated Fatty acids have one or more double bonds between the carbons of the chain.
The 18 C unsaturated fatty acids oleic, linoleic and linolenic acids have 1, 2 and 3 double bonds respectively.
CH3(CH2)7CH = CH(CH2)7COOH General formula of unsaturated fatty acids
Oleic acid CnH2n-2xO2
(where x = number of double bonds)
Arachidonic fatty acid has 4 double bonds.
They have a bend at each double bond which keeps them in liquid form at ordinary temperature.
They are called polyunsaturated fatty acids (PUFA) when they have more than one double bond in them.
They are also called drying oils because they have a tendency to solidify on exposure.
Oils of groundnut, mustard seed, sesame seed and sunflower are rich in unsaturated fatty acids.
The unsaturated fatty acids have lower melting points than saturated fatty acids.
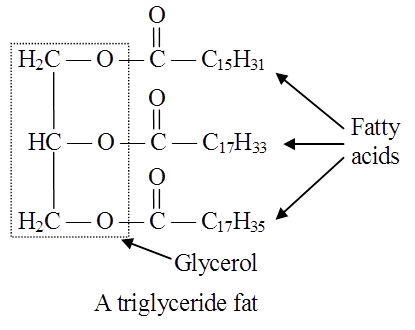
In lipids, fatty acids are usually in the form of esters.
Just as acids and bases react to form salts, similarly organic acids react with alcohol to form esters. Here alcohol is glycerol.
Plants can synthesize all fatty acids.
Animals can not synthesize linoleic, linolenic and arachidonic acid.
These are called essential fatty acids. Their deficiency causes sterility, kidney failure and stunted growth.
Differences between Saturated fatty acids & Unsaturated fatty acids
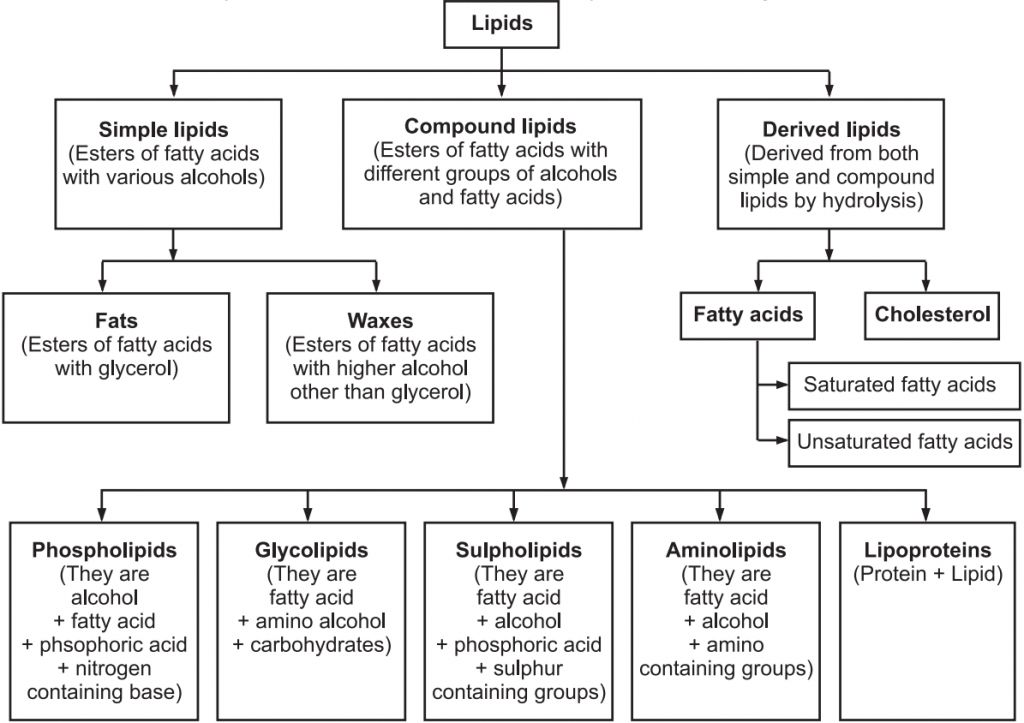
Lipids can be classified as :
1. Simple lipids:
Esters of fatty acids with alcohol.
Simplest alcohol in fats is glycerol (trihydroxypropane).
For example, fats, oils and waxes.
Triglycerides are common in nature.
Fats are esters of fatty acids with glycerol (glycerine).
Each molecule of glycerol can react with three molecules of fatty acids.
Depending on the number of fatty acids that are attached to the glycerol molecule, the esters are called mono-, di-or tri-glycerides.
Fats that are generally liquid at room temperature are called oils.
Oil's are rich in unsaturated fatty acids and consequently have low melting points.
On hydrogenation, the unsaturated fatty acids become saturated and the oil becomes a solid fat ("Vanaspati" and margarine).
(i) Waxes are another class of simple lipids. They are formed by combination of a long-chain fatty acid with a long chain alcohol. Waxes play an important role in protection. They form water-insoluble coatings on hair and skin of animals and stems, leaves and fruits of plants.
(ii) Bees wax is formed from palmitic acid (C16H32O2) and mericyl alcohol (C30H61OH). Bee wax is also called as Hexacosyl palmitate, secreted by worker bees. Lanolin (wool fat), forms a water proof coat around the animal fur.
(iii) Bacteria that cause tuberculosis and leprosy produce a wax (wax-D) that contributes to their pathogenicity.
Cutin is formed by cross esterification and polymerisation of hydroxy fatty acids and other fatty acids without esterification by alcohols other than glycerol. Cuticle has 50 -90% cutin.
Suberin is condensation product of glycerol and phellonic acid. It makes the cell wall impermeable to water.
2. Compound lipids:
These lipids contain an additional group alongwith fatty acids and alcohols e.g. phospholipids, glycolipids, lipoproteins and chromolipids.
(i) Phospholipids:
These are straight chain compounds of glycerol, fatty acids and phosphoric acid.
In these, only two fatty acids are attached to the glycerol molecule and the third hydroxyl group of glycerol is esterified to phosphoric acid instead of fatty acid.
Depending upon the type of phospholipid, this phosphate is also bound to a second alcohol molecule which can be choline, ethanolamine, inositol or serine.
Common phospholipids are lecithin and cephalin.
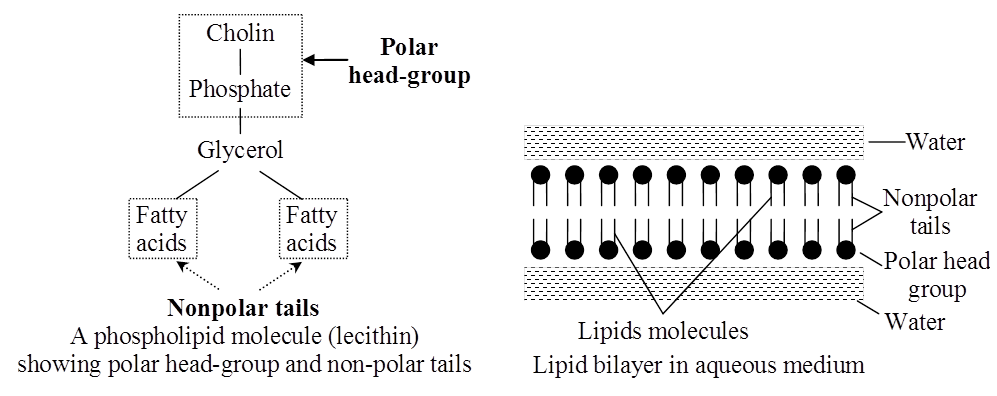
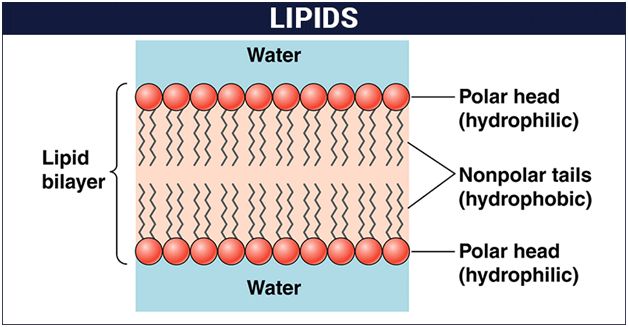
Phospholipids are amphipathic molecules having hydrophilic (water loving) polar region and hydrophobic (water repelling) non-polar regions.
They are the basic constituents of biomembranes.
Many phospholipids arrange themselves in a double layered membrane in aqueous media (lipid bilayer).
Cephalin is found in the brain and acts as insulation material for nerves and also participates in blood coagulation.
Lecithin takes part in cell permeability, osmotic tension and surface conditioning of cells.
The hydrocarbon chains of the fatty acids are the Non-Polar Tails of the molecule.
The phosphate and the nitrogenous/non-nitrogenous groups form the polar Head-Group of the molecule.
Many phospholipid molecules may arrange themselves in a double-layered membrane (Lipid Bilayer) in aqueous media. These have one or more simple sugars.
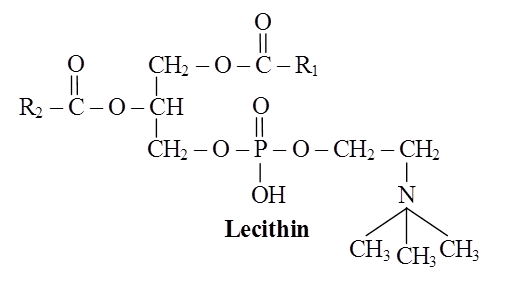
Structure of a phospholipid molecule (R1 and R2 are fatty acids)
(ii) Glycolipids:
They are lipids having sugar residues. Two common glycolipids are cerebrosides and gangliosides.
Composition : Glycolipids contain fatty acids, alcohol sphingosine and sugar such as galactose, glucose etc.
Function : The glycolipids are components of cell membranes, particularly in myelin sheath of nerve fibres and on outer surfaces of nerve cell and in chloroplast membranes.
(iii) Lipoproteins:
Lipoproteins contain lipids (mainly phospholipids) and proteins in their molecules.
Function: Membranes are composed of lipoproteins. Lipids are transported in the blood plasma and lymph as lipoproteins. Lipoproteins occur in the milk and egg yol'k.
(iv) Chromolipids:
These contain pigments such as carotenoids e.g. carotene, vitamin A.
3. Derived lipids –
These are isoprenoid structures e.g. steroids, terpenes, carotenoids, prostaglandins.
(i) Sterols:
Sterols belong to a class of lipids which are not straight chain compounds.
These are composed of fused hydrocarbon rings and a long hydrocarbon side chain.
One of the example is cholesterol.
The cholesterol is found in animals only.
It exists either free or as Cholesterol Ester with a fatty acid.
Cholesterol is also the precursor of hormones such as progesterone, testosterone, estradiol and cortisol.
Another steroid compound, diosgenin produced by the yam plant (Oioscorea) is used in the manufacture of antifertility pills.
(ii) Prostaglandins:
It is a group of hormone-like unsaturated fatty acids which function as messenger substances between cells.
They are derived from arachidonic acid and related C20 fatty acids.
Prostaglandins occur in human seminal fluid menstrual fluid, amniotic fluid and a number of tissues.
They also circulate in blood.
They produce a variety of effects in different organs.
(a) Prostaglandins regulate production of acid in stomach and stimulate contraction of smooth muscles.
(b) They are used to induce labour because they cause uterine contractions.
(c) These can reduce the effect of asthma and gastric acidity. Analgesics like aspirin inhibit prostaglandin synthesis.
(iii) Cholesterol helps in absorption of fatty acids, formation of sex hormones, vitamin D and bile salts. Potato is rich in cholesterol.
(iv) Terpenes are lipid like carbohydrates formed of isoprene units (C5H8)n, e.g., menthol, camphor, carotenoids.
NUCLEOTIDES
(i) This is a group of small complex molecules forming a part of the information transfer system in cells. They are basic units of nucleic acids. They also participate in energy transfer systems
(ii) Nucleotides contain carbon, hydrogen, oxygen, nitrogen and phosphorus.
Each nucleotide is made up of a cyclic nitrogenous base, a pentose and one to three phosphate groups.
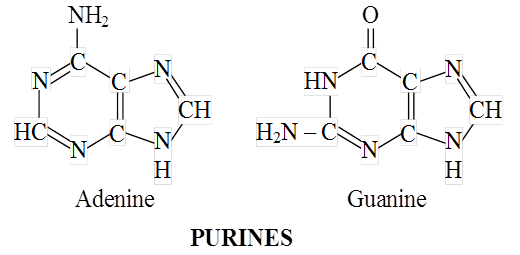
The nitrogenous bases occurring in nucleotides are either a purine or a pyrimidine.
Major purines are adenine and guanine. Thymine, uracil and cytosine are pyrimidines.
The sugar pentose is either ribose or deoxyribose.
The nucleotides are thus called Ribonucleotides or Deoxyribonucleotides.
Examples of ribonucleotides and deoxyribonucleotides are adenylic acid (AMP) and deoxyadenylic acid (d AMP) repetitively.
Ribonucleotides are the basic units of ribonucleic acids (RNA) and deoxyribonucleotides are basic units of deoxyribonucleic acids (DNA).
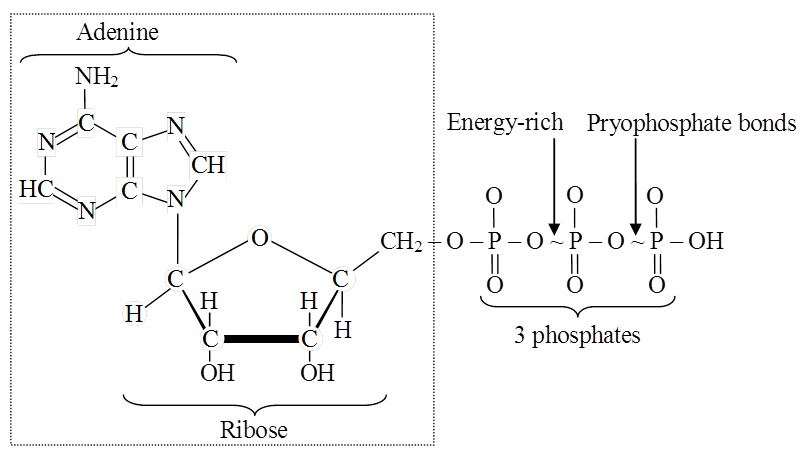
Nucleotides are mono-, di-or tri-phosphate of nucleosides.
For example, adenylic acid or adenosins monophosphate (AMP).
Adenosine disphosphate (ADP) and adenosine triphosphate (ATP) are higher adenine nucleotides.
Nucleotides with more than one phosphate group are called higher nucleotides, e.g., ATP and ADP. likewise, other purines and pyrimidines can also form higher nucleotides.
(iii) Higher nucleotides of purines and pyrimidines occur in the free state, e.g., ATP, ADP. Their third and second phosphate bonds can release about 8 kcal or more of free energy per mol on hydrolysis. This far exceeds the energy released on hydrolysis of most other covalent bonds. Therefore, these phosphate bonds of higher nucleotides are called High-Energy Bonds.
Nucleotides of the vitamins nicotinamide and riboflavin occur either freely or in combination with specific proteins, thus work as coenzymes. They do not participate in the formation of nucleic acids. Instead, they act along with oxidising enzymes and participate in oxidation reactions occurring in the cell.
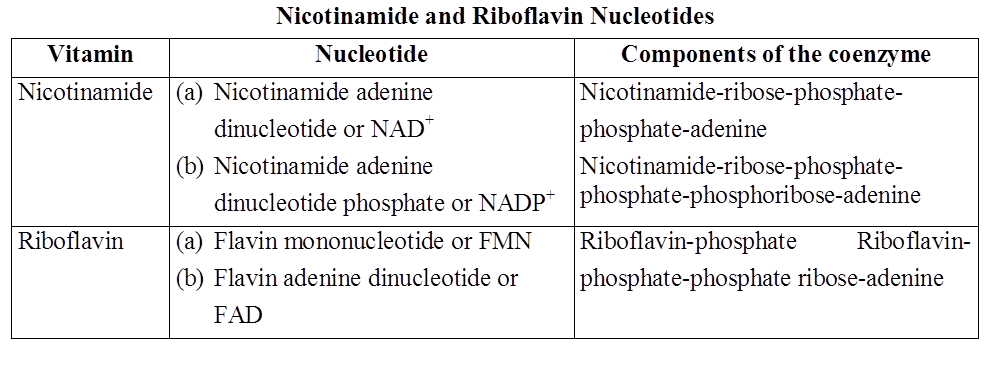
Functions of Nucleotides
Purine and pyrimidine nucleotides polymerise to form nucleic acids.
Higher purine and pyrimidine nucleotides, particularly ATP, store energy in their high-energy phosphate bonds.
They are formed during photosynthesis and respiration.
Hydrolysis of the phosphate bonds of ATP releases their bond energy for driving energy-dependent reactions and processes.
Nicotinamide and riboflavin nucleotides act as coenzymes of oxidising enzymes.
Lipids
Lipids are organic molecules containing hydrogen, carbon, and oxygen atoms that provide the structural and functional foundation for living cells. Because water is a polar molecule, these organic compounds are nonpolar molecules that are only soluble in nonpolar solvents and insoluble in water. These molecules are produced in the human liver and can be found in the oil, butter, whole milk, cheese, fried foods, and some red meats.
Structure of Lipids
Lipids are fatty acid polymers with a long, non-polar hydrocarbon chain and a short polar area that contains oxygen.Lipids are often insoluble in water. It's possible that they're just simple fatty acids. A carboxyl group is connected to the R group in a fatty acid. The R group could be methyl (–CH3), ethyl (–C2H5), or a combination of the two (1 carbon to 19 carbons). Palmitic acid, for example, comprises 16 carbons, including the carboxyl carbon. Arachidonic acid, including carboxyl carbon, has 20 carbon atoms.Saturated fatty acids have no double bonds, while unsaturated fatty acids have one or more C=C double bonds. Glycerol, which is trihydroxy propane, is another simple lipid. Glycerol and fatty acids are found in many lipids. The fatty acids are esterified with glycerol. Monoglycerides, diglycerides, and triglycerides are the three types of lipids. Based on their melting point, these are also known as fats and oils. Oils (e.g., gingely oil) have a lower melting point and hence remain as oil in the winter. Phosphorus and a phosphorylated organic molecule are found in some lipids. Phospholipids are what they're called. They're located in the membranes of cells. One example is lecithin. Lipids with more complicated structures are found in some tissues, particularly brain tissues.
Listed below are some important characteristics of Lipids.
1. Lipids are oily or greasy nonpolar molecules, stored in the adipose tissue of the body.
2. Lipids are a heterogeneous group of compounds, mainly composed of hydrocarbon chains.
3. Lipids are energy-rich organic molecules, which provide energy for different life processes.
4. Lipids are a class of compounds characterized by their solubility in nonpolar solvents and insolubility in water.
5. Lipids are significant in biological systems as they form a mechanical barrier dividing a cell from the external environment known as the cell membrane.
Classification of Lipids:
Lipids serve a critical role in our bodies. They are a component of the cell membrane's structure. They aid in the production of hormones and provide energy to our bodies. They aid in appropriate meal digestion and absorption. If we eat them in the right amounts, they constitute a nutritious element of our diet. They play a vital function in signaling as well.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
