- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Carbohydrates
Carbohydrates are mainly compounds of carbon, hydrogen and oxygen.
Carbohydrates are so called because in most of them, the proportion of hydrogen and oxygen is the same as in water (H2O) i.e., 2 : 1.
These are also known as saccharides (compounds containing sugar).
Carbohydrates are produced by green plants during photosynthesis.
These constitute about 80% of the dry weight of plants.
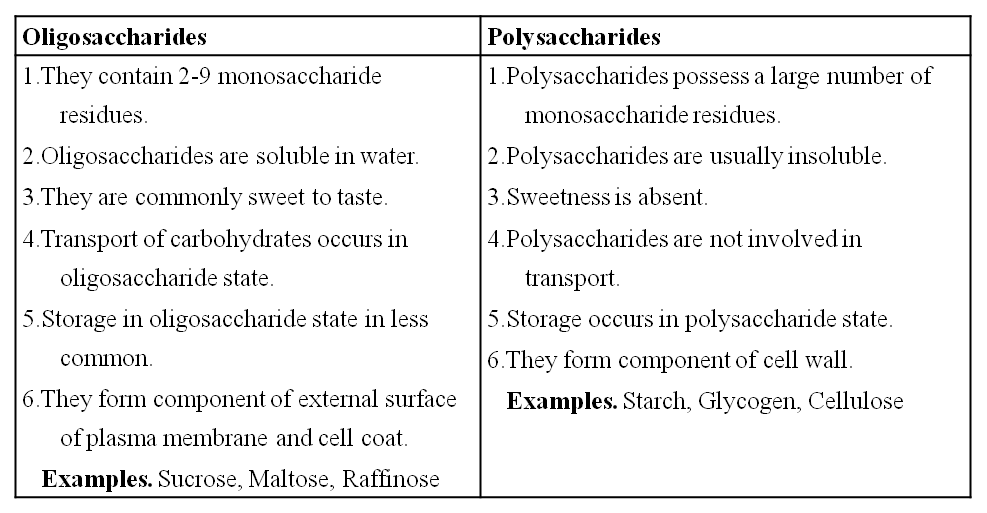
Carbohydrates are divided into 3 main classes -monosaccharides, oligosaccharides and polysaccharides.
1. Monosaccharides
(i) These are single saccharide units with CnH2nOn general formula which cannot be hydrolysed further into still smaller carbohydrates. These are composed of 3-7 carbon atoms and are classified according to the number of C atoms as trioses (3C), tetroses (4C), pentoses (5C), hexoses (6C) and heptoses (7C). Of these, pentoses and hexoses are most common. Monosaccharides are important as energy sources and as building blocks for the synthesis of large molecules.
(ii) All monosaccharides are either aldoses or ketoses. Simplest monosaccharides include trioses e.g., glyceraldehyde and dihydroxyacetone.
(iii) Tetroses (e.g., erythrose) are rare. Erythrose takes part in the synthesis of lignin and anthocyanin pigments.
(iv) Ribose, ribulose, xylulose and arabinoses are pentoses. Xyluloses and arabinoses polymerise to form xylans and arabans which are cell wall material.
(v) Glucose, fructose, mannose, galactose are hexoses. These are white, sweet-tasting, crystalline and extremely soluble in water.
(vi) Glucose is called universal sugar and is also known as dextrose or grape sugar or corn sugar.
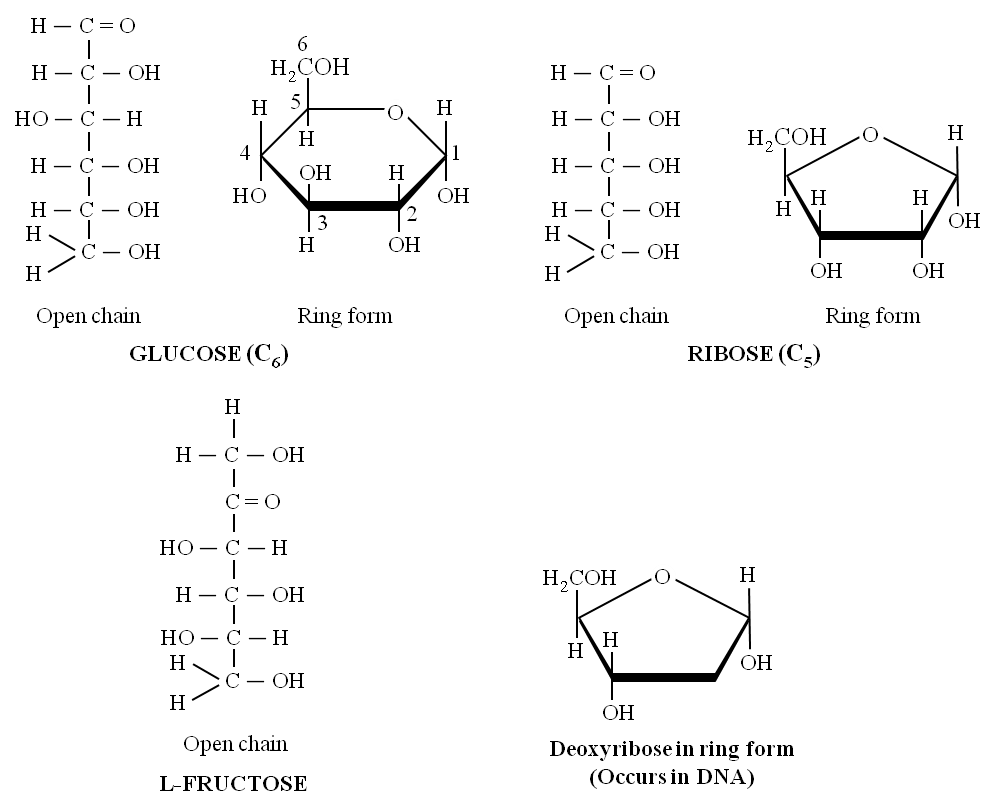
(vii) Fructose is called fruit sugar and is also known as levulose. It is the naturally occurring sweetest sugar. Honey has two sugars -Dextrose and Levulose.
(viii) Heptoses have 7 carbon atoms per molecule of sugar with general formula C7H14O7 e.g., sedoheptulose. It is an intermediate of respiratory and photosynthetic pathways.
Pentoses and hexoses of monosaccharides occur in solid forms i.e., open chain and ring chain. There are two types of ring chains i.e.,
(a) pyranose ring, which has hexagonal shape with 5 carbon atoms and one oxygen atom and
(b) furanose ring, which has pentagonal shape with 4 carbon atoms and one oxygen atom.
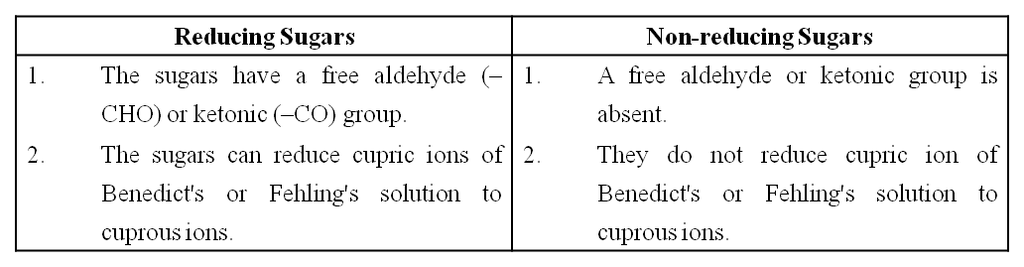
(ix) Monosaccharides have 'free' aldehyde or ketone group which can reduce Cu++ to Cu+. Hence, these are also called reducing sugars.
(x) Monosaccharides have two important chemical properties.
(a) Sugars having a free aldehyde or ketone group can reduce Cu++ to Cu+. These are called reducing sugars. This property is the basis of Benedict's test and Fehling's test to detect the presence of glucose in urine.
(b) The aldehyde or ketone group of monosaccharide can react and bind with an alcoholic group of another organic compound to join the two compounds together. This bond is called the glycosidic bond. This bond can be hydrolysed to give the original reactants.
Differences between Reducing and Non-reducing Sugar
Concept Builder
Derived Monosaccharides
(i) Deoxysugar -Loss of oxygen atom at 2nd carbon of ribose, yields deoxyribose, a constituent of DNA.
(ii) Amino sugar -Monosaccharides having an amino group e.g. glucosamine, galactosamine
(iii) Sugar acid -e.g., Ascorbic acid, glucuronic acid, galacturonic acid.
(iv) Sugar alcohol -e.g., glycerol and mannitol (present in brown algae).
3. Oligosaccharides : They are condensation product of (2-9) monosaccharides. These include diasaccharides, trisaccharides, tetrasaccharides, hexasaccharides, heptasaccharides etc.
Differences between Oligosaccharides and Polysaccharides
(a) Disaccharides:
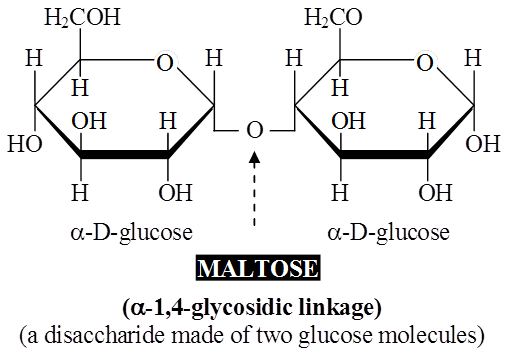
These are formed by condensation reactions between two monosaccharides (usually hexoses).
The bond formed between two monosaccharides is called a glycosidic bond.
It normally forms between C-atoms 1 and 4 of neighbouring units (1, 4 bond).
Once linked, the monosaccharide units are called residues.
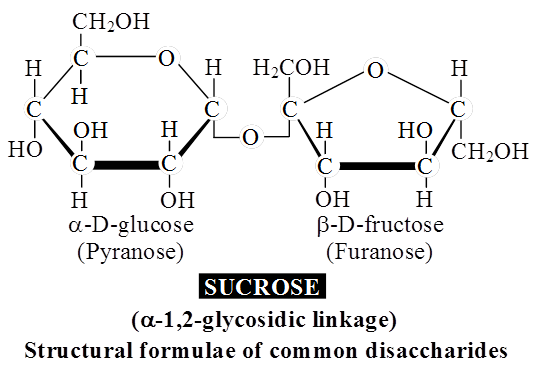
A molecule of sucrose is formed from a molecule of glucose and one of fructose.
Sucrose is the storage product of photosynthesis in sugarcane and sugarbeet.
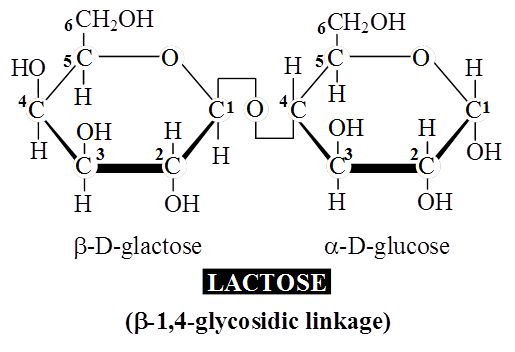
Lactose or milk sugar is found in human milk and cow's milk.
It is formed from one glucose molecule and one of galactose.
Maltose or malt sugar is formed from two molecules of glucose during germination of starchy seeds.
Maltose and lactose are reducing disaccharides.
Sucrose does not reduce Cu++ to Cu+, hence sucrose is a non-reducing sugar.
(b) Trisaccharides:
Sugars composed of 3 monosaccharide units are called trisaccharides (e.g. raffinose).
Raffinose is a common trisaccharide found in plants.
Upon hydrolysis, it yields one molecule each of glucose, fructose and galactose.
Larger oligosaccharides are attached to the cell membrane and enable the cell-cell recognition due to their presence.
They also take part in antigen specificity.
4. Polysaccharides
These are polymers of monosaccharides and are branched or unbranched linear molecular chains.
These are insoluble carbohydrates and are considered to be non-sugars.
Starch, glycogen, cellulose, pectin, hemicellulose, inulin are polysaccharides.
Body cells store carbohydrates as polysaccharides since these are easy to store and can be easily converted back into simple carbohydrates upon hydrolysis. These are in more condensed form and they have high molecular weight. These cannot pass through the plasma membrane.
Polysaccharides are of two types :
(i) Homopolysaccharides - consist of only one type of monosaccharide monomer e.g. starch, glycogen and cellulose, fructan, xylan, araban, galactan.
(ii) Heteropolysaccharides - consist of more than one type of monosaccharide monomer e.g. chitin, agar, arabanogalactans, arabanoxylans etc.
Polysaccharides are of three main types -storage (e.g. starch and glycogen), structural (e.g. chitin, cellulose) and mucopolysaccharides (e.g. keratan sulphate, chondroitin sulphate, hyaluronic acid, agar, alginic acid, carrageenin and heparin).
(a) Storage Polysaccharides
Food-Storage Polysaccharides: Starch is found abundantly in rice, wheat and other cereal grains legumes, potato, tapioca and bananas.
It is formed during photosynthesis and serves as an energystoring material.
Glycogen found in liver and muscles stores energy in mammals.
Storing carbohydrates in the form of polysaccharides has two advantages.
During their formation, many molecules of water are removed from monosaccharides.
This helps in condensing the bulk to be stored.
Unlike small carbohydrates, polysaccharides are relatively easy to store.
When necessary, polysaccharides are broken down by enzymes for the release of energy.
Starch
Starch, glycogen and inulin are reserve food materials.
Starch is a polymer of a-D-glucose. It is the major reserve food in plants.
Starch has two components -amylose (an unbranched polymer) and amylopectin (a branched polymer).
Amylopectin: Consists of 2000 -200,000 glucose molecules forming straight chain and shows branching (after 25 glucose units). Branching point has , 1-6 glycosidic linkage.
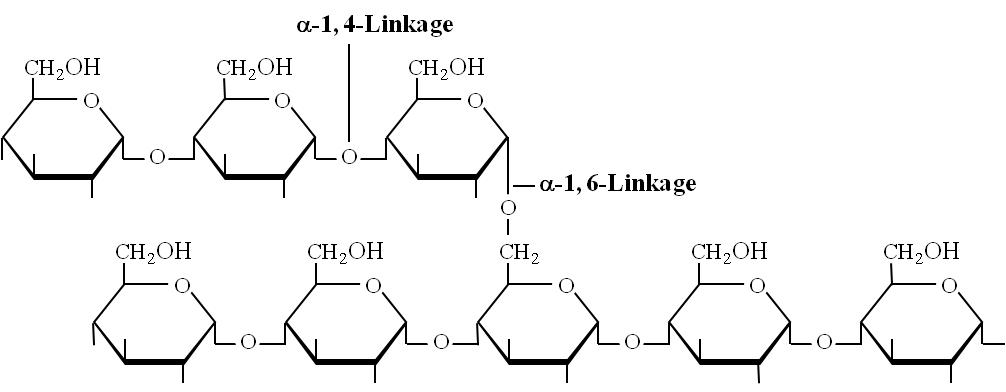
Amylopectin (branched polysaccharide)
Amylose: Consists of , 1-4 glycosidic linkage between -D glucose molecules. It is a straight chain of 200 -1000 glucose units. Starch forms helical secondary structures, each turn consists of 6 glucose units.
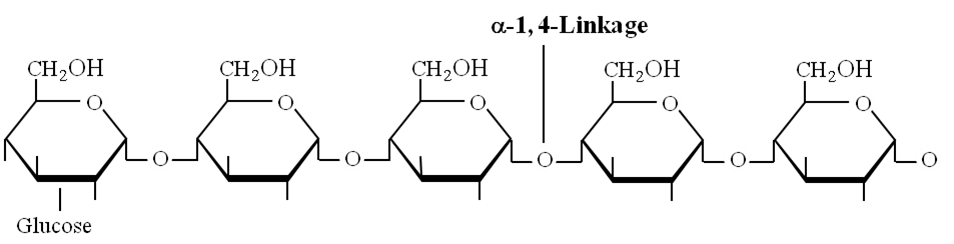
Structure of amylose showing -1, 4 linkage
Concept Builder
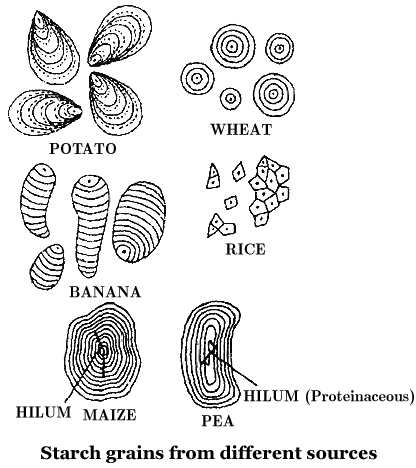
Starch molecules accumulate in the form of layers (stratifications) around a shifting organic centre (hilum) to form starch grains.
Hilum is made up of protein. In eccentric starch grains, hilum lies on one side.
These are found in potatoes.
In concentric starch grains, hilum is present in the centre.
These are found in wheat, maize, pea.
Dumb-bell shaped starch grains are found in the latex of Euphorbia.
Starch grains with single hilum are called simple (e.g. maize) but those with more than one hilum are called compound (e.g. potato, rice).
Starch turns blue with iodine as the helices in starch hold I2.
(ii) Glycogen: Glycogen is the animal equivalent of starch, many fungi also store it. Glycogen turns red-violet with iodine.
It consists of 30,000 glucose units joined by , 1-4 bonds, much more branched than starch. Branch point has , 1-6 linkages and branching occurs after 10-14 glucose units.
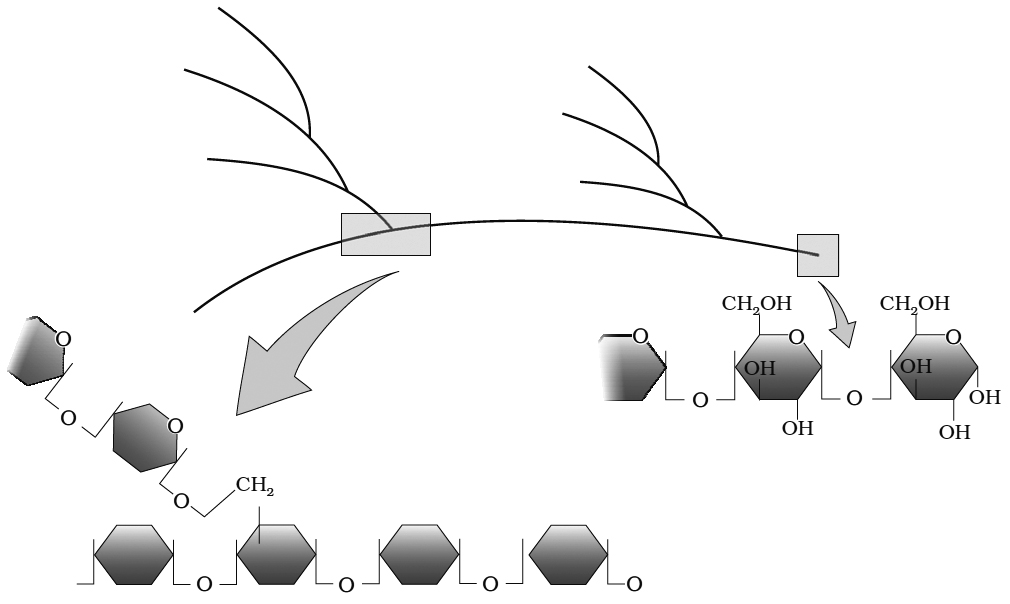
Diagrammatic representation of a portion of glycogen
(iii) Inulin: It is an unusual polysaccharide and polymer of fructose. It is stored particularly in roots and tubers of the family Compositae e.g. Dahlia tubers.
(b) Structural Polysaccharides Cellulose (Hexosan polysaccharide) :
Cellulose is the main structural unbranched homopolysaccharic of plants.
One molecule of cellulose has about 6000 -glucose residues.
Cotton fibres contain the largest amount (90 percent) of cellulose among natural materials.
Wood contains between 25 to 50 percent cellulose, the rest being hemicellulose and lignin.
Fibres of cotton, linen and jute are used for textile and ropes.
The artificial fibre Rayon is manufactured by dissolving cellulosic materials in alkali and by extruding and coagulating the filaments.
By treatment with other chemicals, cellulose is converted into Cellulose Acetate (used in fabrics, cellulosic plastics and shatter-proof glass), Cellulose Nitrate (used in propeliant explosives) and Carboxymethyl Cellulose (added to ice creams, cosmetics and medicines to emulsify and give a smooth texture).
Cellulose can be hydrolysed to soluble sugars.
Microbes can then convert these sugars to form ethanol, butanol, acetone, methane and other useful chemicals.
Cellulose is unbranched homopolysaccharide of -glucose.
Cellulose is the most abundant carbohydrate in biosphere.
Cellulose is produced by plants and is used for building cell walls. Cellulose is the most abundant organic compound in the biosphere.
Wood and cotton contain large quantities of cellulose.
Chitin is a polysaccharide found in the exoskeleton of insects, crabs and prawns.
Chitin is similar to cellulose in many ways except that its basic unit is not glucose, but a similar molecule that contains nitrogen (N-acetylglucosamine).
Although chitin is soft and leathery, it becomes hard when impregnated with calcium carbonate or certain proteins.
The insolubility of these polysaccharides in water helps to retain the form and strengthens the structure of organisms.
Pectin and hemicellulose: Pectin and hemicelluloses are structural polysaccharides.
Pectins are made up of arabinose, galactose and galacturonic acid.
Pectic acid is an acidic polysaccharide of methyl ester of D-galacturonic acid.
Middle lamella which binds the cells together is composed of calcium pectate.
Due to this substance, water absorption capacity of cell wall is increased.
Fruit walls contain high percentage of pectin.
During ripening, pectin breaks down into simple sugars resulting in the sweetening and loosening of fruits.
Hemicellulose is a mixture of D-xylose linked by 1-4 glycosidic bond.
Xylans, arabans, galactans are hemicelluloses. Food such as dates -Phoenix have hemicellulose as reserve food.
(c) Mucopolysaccharides
The slimy substances produced by plants are called mucilages.
When you soak the seeds of isabgol (Plantago ovata) or cut the fruit of okra (bhindi), you will notice the presence of a slimy substance.
Mucilages are polysaccharides formed from galactose and mannose.
Many seaweeds yield mucilages of commercial value such as agar, alginic acid and carrageenin.
Mucopolysaccharides are found in cell walls of bacteria and in the connective tissues of animals, as well as in body fluids.
These bind proteins in cell walls and connective tissue and water in interstitial spaces thereby providing lubrication in ligaments and tendons.
The vitreous humor of the eye and synovial fluid also contain mucopolysaccharides.
Hyaluronic acid is found in connective tissue and in cell walls.
Keratin sulphate and chondroitin sulphate occur in cartilage, cornea and the skin and impart strength and flexibility to them.
Keratan sulphate - consists of acetyl glucosamine, galactose and sulphuric acid, provides strength and flexibility to skin and cornea.
Hyaluronic acid -consists of D-glucuronic acid and N-acetyl glucosamine, present in the vitreous humor of eye, synovial fluid and cerebrospinal fluid etc.
Heparin is a polymer of sulfated glucosamine and sulfated iduronic acid.
It is an anticoagulant present in human blood.
Husk of Plantago ovata and mucilage of Aloe barbadensis are medicinally used.
Agar, alginic acid carrageenin are obtained from marine algae.
Artificial silk is polysaccharide prepared from rayon.
Carbohydrates
Carbohydrate is a group of organic compounds occurring in living tissues and foods in the form of starch, cellulose, and sugars. It is one of the three micronutrients via which a human body obtains energy. The properties of carbohydrate biology include carbon, hydrogen, and oxygen atoms at their chemical level. Cn(H2O)n is the generic formula for all carbohydrates. This formula is only valid for simple sugars, which are made up of the same amount of carbon and water.
There are two types of carbohydrates, simple and complex. This division is primarily based on their chemical structure along with their degree of polymerization.
Simple Carbohydrates: Simple carbohydrates carry one or two molecules of sugar. Such examples of carbohydrates are found abundantly in dairy products, refined sugar, etc. Since these carbohydrates do not comprise any fiber, vitamin, or mineral, they are regarded as empty calories. Simple carbohydrates can be further divided into three categories. These are as follows:
Monosaccharides: Carbohydrates consisting of one sugar molecule are called monosaccharides. Monosaccharides can be further classified based on the number of carbon atoms. These are trioses, tetroses, pentoses, hexoses, and heptoses. One of the most significant monosaccharides is glucose. The following are the two most frequent methods for preparing glucose. Sucrose is converted to glucose and fructose when it is cooked with dilute acid in an alcohol solution. From Starch, Glucose can also be made by hydrolyzing starch and boiling it with weak sulphuric acid at 393 degrees Fahrenheit under high pressure. Glucose, commonly known as dextrose and aldohexose, is abundant on the planet.
Disaccharides: Two monosaccharides combine to form a disaccharide. Sucrose, Lactose, and Maltose are some of the prime examples of this carbohydrate.
Oligosaccharides: Carbohydrates consisting of 2-9 monomers are classified as oligosaccharides.
The term “monosaccharide” refers to a carbohydrate derivative possessing a single carbon chain; “disaccharide” and “trisaccharide” refer to molecules containing two or three such monosaccharide units joined together by acetal or ketal linkages. “Oligosaccharide” and “polysaccharide” refer to larger such aggregates, with “a few” and many monosaccharide units, respectively. Current usage seems to draw the distinction between “few” and many at around 10 units.
Complex Carbohydrate: Complex carbohydrates are made up of two or more molecules of sugar. Such carbohydrates are found abundantly in food items like corn, lentils, peanuts, beans, etc. Complex carbohydrates are also known as polysaccharides as they are formed due to polymerization.
Polysaccharides are another macromolecule family found in the acid-insoluble pellet. Polysaccharides are lengthy sugar chains. They're threads (literally, cotton threads) made up of various monosaccharides as building blocks. cellulose, for example, is a polymeric polysaccharide made up of only one type of monosaccharide, glucose. Cellulose is a homopolymer, which means it is made up of only one type of molecule. Starch is a type of this that is found in plant tissues as a source of energy. Glycogen is a different type of carbohydrate found in animals. Inulin is a fructose polymer. The right end of a polysaccharide chain (such as glycogen) is known as the reducing end, while the left end is known as the non-reducing end. It has branches. Secondary helical structures are formed by starch.In fact, the helical part of starch can retain I2 molecules. The colour of starch-I2 is blue. Because cellulose lacks complex helices, it is unable to retain I2.
Cellulose is the main component of plant cell walls. Cellulosic paper is created from plant pulp and cotton fibre. In nature, there exist more complicated polysaccharides. Amino-sugars and chemically modified sugars are used as building blocks (e.g., glucosamine, N-acetyl galactosamine, etc.). Arthropod exoskeletons, for example, contain a complex polymer called chitin. The majority of these complex polysaccharides are homopolymers.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
