- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
PROTEINS
Berzelius coined the term protein. Proteins are hetero polymers of amino acids.
Two amino acids can join through amino group of one and carboxylic group of the other forming an anhydro bond (CO-NH linkage) also known as peptide bond by loss of water molecule.
A protein is a heteropolymer and not a homopolymer.
Collagen is the most abundant protein in animal world and Rubisco (Ribulose biphosphate carboxylase oxygenase) is the most abundant protein in the whole biosphere.
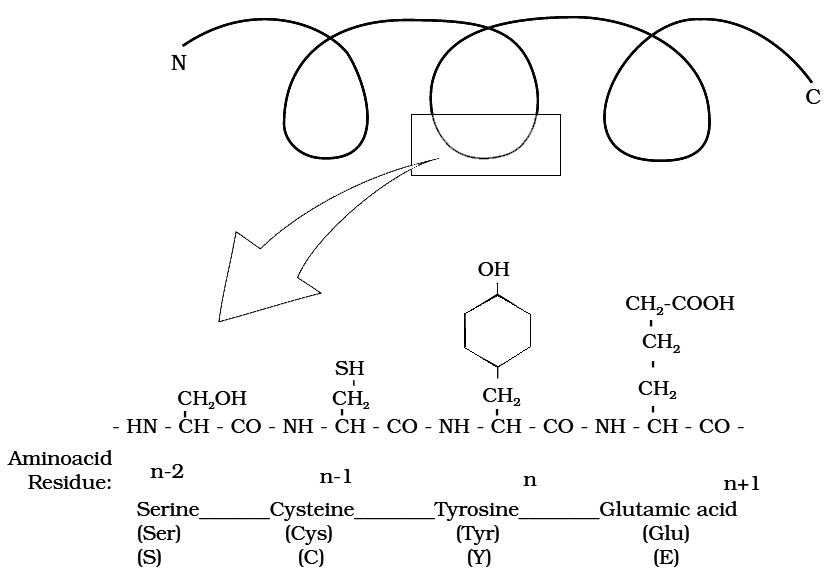
Primary structure of a portion of a hypothetical protein.
N and C refer to the two termini of every protein. Single letter codes and
three letter abbreviations of amino acids are also indicated.
Structure of Proteins :
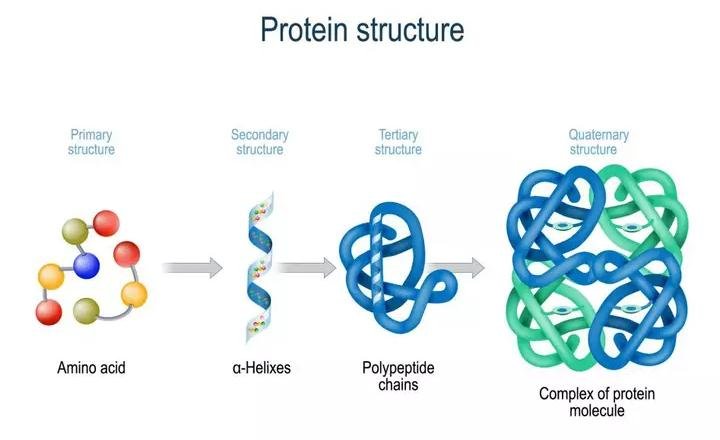
The four levels of protein structure are:
1. Primary Structrure :
The sequence of amino acids in polypeptide chain gives the protein its Primary Structure.
The primary structure is very important as it determines the specificity of protein but does not make a protein functional.
To be functional the protein must have a particular 3-dimensional structure (conformation).
A functional protein contains one or more polypeptide chains.
The sequence of amino acids in the chain determines where the chain will bend or fold and where the various lengths will be attracted to each other.
2. Secondary Structure :
Through the formation of hydrogen bonds, peptide chains assumes a Secondary Structure.
When a chain is arranged like a coil it is called an Helix.
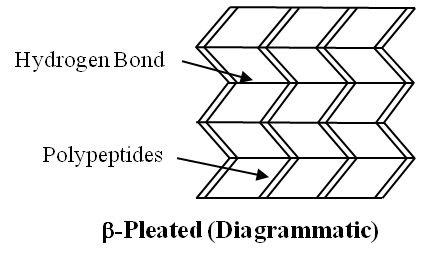
When two or more chains are joined together by intermolecular hydrogen bonds, the structure is called Pleated Sheet.
Helical structure is found in keratin of hair and pleated structure found in silk fibres.
Each protein has a specific secondary structure also.
(a) It generailly takes the form of an extended spiral spring, the -helix, whose structure is maintained by many hydrogen bonds which are formed between adjacent -CO and -NH groups. The H atom of the NH group of one amino acid is bonded to the O atom of the CO group three amino acids away. A protein which is entirely helical is keratin.
(b) The other type of secondary structure is called -pleated sheet. Here, two or more chains are joined together by intermolecular hydrogen bonds as in silk fibres.
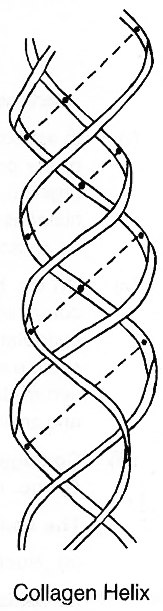
(c) A special secondary structure is observed in collagen or tropocollagen helix which has three strands or polypeptides coiled around one another. The coil is strengthened by the establishment of hydrogen bond between -NH group of glycine residue of each strand with -CO group of the other two strands. Locking effect is due to the proline and hydroxyproline.
3. Tertiary structure :
Usually, the polypeptide chain bends and folds extensively and forms a compact 'globular' shape to obtain functional conformation. This is termed as the tertiary structure.
 Various types of bonds or interactions found during coiling of polypeptide
Various types of bonds or interactions found during coiling of polypeptide
In a large protein like haemoglobin, or in case of an enzyme, the molecule undergoes further folding and coiling to attain functional conformation.
The coils and folds of the protein molecule are so arranged as to hide non-polar amino acid side chains inside and expose the polar side chains.
The 3-dimensional conformation of a protein brings distant amino acid side chains closer.
The active sites of proteins such as enzymes are thus formed.
The conformation of proteins is easily changed by pH, temperature and chemical substances and hence the function of proteins is liable and subject to regulation.
4. Quarternary strucrure :
Many highly complex proteins consist of an aggregation of polypeptide chains held together by hydrophobic interactions and hydrogen and ionic bonds.
Their precise arrangement constitutes the quaternary structure.
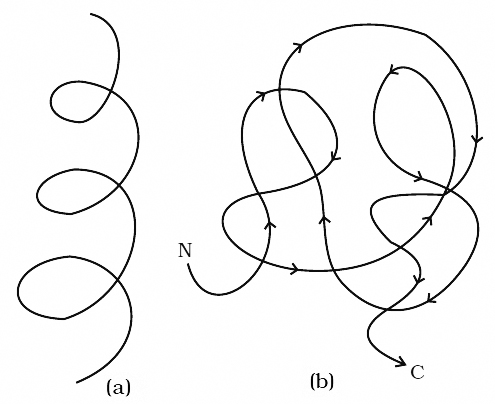
Cartoon showing: (a) A secondary structure and (b) A tertiary structure of proteins
In aqueous media, proteins carry both cationic and anionic groups on the same molecule.
The ionic state of the protein depends on the pH of the medium.
A protein, rich in basic amino acids like lysine and arginine, exists as a cation and behaves as a base at the physiological pH of 7.4 (Basic Protein) e.g., histones of nucleoproteins.
Similady, a protein with acidic amino acids exists as an anion and behaves as an acid e.g., most blood proteins (Acidic Proteins).
Types of Proteins :
On the basis of constitution, proteins are classified as simple or conjugated.
1. Simple Protein :
Simple Proteins are composed of amino acids only.
Some are small, globular molecules mostly soluble in water and not coagulated by heat (e.g., histones).
As the size of the protein molecule increases, it becomes less soluble and its heat-coagulability increases.
For example, larger globular proteins (like egg albumin, serum globulins and glutelins of wheat or rice) are coagulated by heat.
Fibrous proteins have long molecures and are insoluble in water (e.g., keratin of skin and hair and collagen of connective tissues).
2. Conjugated proteins :
Conjugated Proteins are formed by binding of a simple protein with a non-protein called tile Prosthetic Group. e.g., Nucleoproteins have nucleic acids as prosthetic group.
The conjugated proteins are of following types :
(a) Nucleoproteins (prosthetic group-nucleic acid) e.g. protamines
(b) Metalloproteins (prosthetic group-metals) e.g. haemoglobin
(c) Chromoproteins (prosthetic group-pigment) e.g. cytochromes
(d) Phosphoproteins (prosthetic group-phosphoric acid) e.g. Casein of milk.
(e) Lipoproteins (prosthetic group-lipids) e.g. chylomicrons, HDL, LDL etc.
(f) Glycoproteins (prosthetic group-carbohydrates) e.g. mucins
Glycoproteins (and glycolipids) play an important role in cell recognition.
The specificity of this recognition depends upon the particular sequence of sugars in carbohydrate portions.
Ribulose biphosphate carboxylase (an enzyme) present in large amounts in chloroplast stroma is the world's most common protein.
Storage Proteins include albumin of egg and those that occur in seeds (glutelin of wheat). Prolamines are storage proteins.
Protamines are basic proteins associated with DNA of chromosomes, they are rich in lysine and arginine.
P-proteins are involved in the transport of organic compounds through phloem.
Keratin and fibroin form protective structures.
Antibodies are defence proteins.
Snake venom, ricin of castor and bacterial toxins are proteinaceous in nature.
Actin and myosin are essential for muscle contraction.
Microtubules have tubulin protein.
Haemoglobin and myoglobin are transport proteins.
Ovalbumin and glutelin are storage proteins present in cereals. Ferretin is iron storing protein of animal tissues. The type of prolamines and glutelins found in wheat are gliadin and glutenin.
Insulin and parathormone are proteinaceous hormones.
Fibrinogen and thrombin are blood clotting prbteins.
Rhodopsin and iodopsin are photoreceptor pigments. These are present in rods and cones of retina and are proteins.
Proteins having all essential amino acids are called first class proteins.
Monellin, a protein, is the sweetest chemical obtained from an African berry.
Cheese is a denatured protein.
Resilin -is a perfectly elastic protein found in wings of some insects.
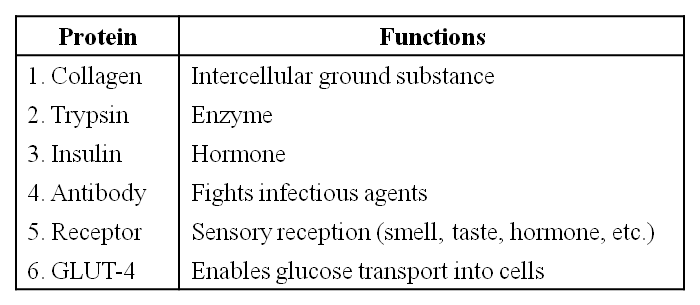
Some proteins and their Functions
Proteins
Polypeptides are the building blocks of proteins. They are peptide bonds that connect linear chains of amino acids. A polymer of amino acids makes up each protein. A protein is a heteropolymer, not a homopolymer because there are 20 different types of amino acids. A homopolymer is made up of only one type of monomer that repeats 'n' times. Specific amino acids are necessary for human health and must be obtained through our food. As a result, necessary amino acids are obtained from food proteins. As a result, amino acids can be classified as either essential or non-essential. The latter are those that our bodies can produce, whereas essential amino acids must be obtained through our diet.Proteins have a variety of activities in living creatures, including transporting nutrients across cell membranes, fighting pathogenic organisms, acting as hormones, and enzymatic reactions. The most abundant protein in the animal kingdom is collagen, and the most abundant protein in the biosphere is Ribulose bisphosphate Carboxylase-Oxygenase (RuBisCO).
Structure of Proteins:
Proteins are heteropolymers made up of strings of amino acids, as previously stated. In different situations, the structure of molecules means different things. Physicists visualize molecular structures in three dimensions, while biologists explain protein structure on four levels. The primary structure of a protein is the sequence of amino acids, or the positional information in a protein — which amino acid is the first, which is the second, and so on.A protein can be visualized as a line, with the initial amino acid on the left end and the last amino acid on the right.
N-terminal amino acid is another name for the first amino acid. The C-terminal amino acid is the last one in the chain. As an extended stiff rod, a protein thread does not exist throughout. The thread is folded into a helix shape (similar to a revolving staircase). Naturally, only a piece of the protein thread is organized in a helix shape. Only right-handed helices are found in proteins. In what is known as the secondary structure, various portions of the protein thread are folded into other configurations.
Furthermore, the lengthy protein chain folds in on itself like a hollow woollen ball, forming the tertiary structure. This allows us to see a protein in three dimensions. Proteins' tertiary structure is required for many of their biological functions. Some proteins are made up of several polypeptides and subunits. The architecture of a protein, also known as the quaternary structure of a protein, is the way these individual folded polypeptides or subunits are arranged with regard to one other (e.g., a linear string of spheres, spheres placed one upon another in the form of a cube or plate, etc.).Adult human haemoglobin consists of 4 subunits. Two of these are identical to each other. Hence, two subunits of α type and two subunits of β type together constitute the human haemoglobin (Hb).
Nature of bond linking monomers in a polymer
A peptide bond is produced when the carboxyl (-COOH) group of one amino acid combines with the amino (-NH2) group of the next amino acid with the elimination of a water molecule in a polypeptide or protein (the process is called dehydration). Individual monosaccharides in a polysaccharide are joined by a glycosidic bond. Dehydration also contributes to the formation of this link. Two carbon atoms from two neighboring monosaccharides make a peptide bond. A phosphate moiety of a nucleic acid connects the 3'-carbon of one sugar of one nucleotide to the 5'-carbon of the sugar of the next nucleotide. An ester link exists between the phosphate and the sugar's hydroxyl group.The phosphodiester bond is named after the fact that there is one such ester bond on each side.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
