- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Solution and its properties
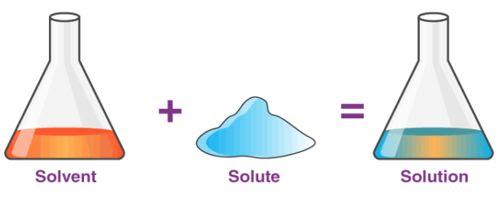
A solution is a homogeneous mixture of two or more substances. Ex: Lemonade, soda water etc
A solution has two components:
(i) Solvent
(ii) Solute
(i) Solvent: The component of the solution that dissolves the other component in it (usually the component present in larger amount) is called the solvent.
(ii) Solute: The component of the solution that is dissolved in the solvent (usually present in less quantity) is called the solute.
Properties of Solution:
1. A solution is a homogeneous mixture.
2. The particles of a solution are smaller than 1 nm
(10-9 ) in diameter which cannot be seen by naked
eyes.
3. They do not scatter a beam of light passing through the solution that is they don’t show tyndall effect. So, the path of light is not visible in a solution.
4. The solute particles cannot be separated from the mixture by the process of filtration.
5. The solution is stable and solute particles do not settle down when left undisturbed.
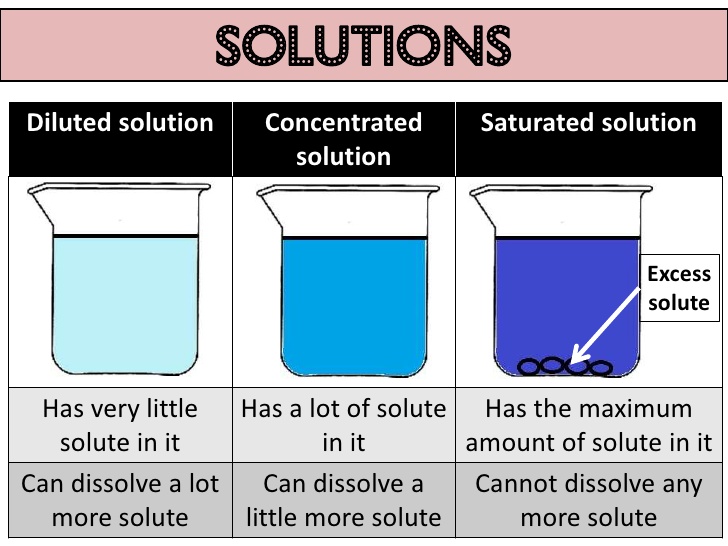
Concentration of a solution
(i) Saturated solution: When no more amount of solute can be dissolved in a solution at a give temperature, it is called a saturated solution.
(ii) Unsaturated solution: When more amount of solute can be dissolved in a solution at a give temperature, it is called a saturated solution.
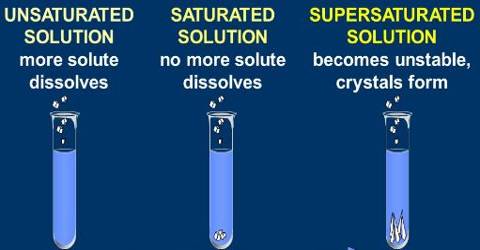
(ii) Solubility: The amount of the solute present in the saturated solution at the given temperature
called its solubility.
The concentration of a solution is the amount of solute present in a given amount (mass or volume) of solution. Also, the amount of solute dissolved in a given mass or volume of solvent is called concentration of solution.
Concentration of solution = Amount of solute/Amount of solvent or Amount of solute/Amount in
solution (Here, amount means mass or volume).
Two methods of finding concentration of solution:
(i) Mass by mass percentage of a solution = (Mass of solute/Mass of solution) ×100
(ii) Mass by volume percentage of a solution = (Mass of solute/Volume of solution) ×100

 Vaishnav Publication
Vaishnav Publication
 ACERISE INDIA
ACERISE INDIA
