- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Ions
→ An ion may be defined as an atom or group of atoms having positive or negative charge.
→ Some positively charged ions : Na+ , K+ , Ca2+ , Al3+
→ Some negatively charged ions : Cl- (chloride ion), S2- (sulphide ion), OH-(hydroxide ion),
SO42- (sulphate ion)
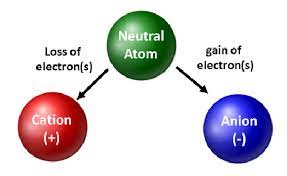
• We can classify ions in two types:
(i) Simple ions
Mg2+ (Magnesium ion)
Na+(Sodium ion)
Cl-(Chloride ion)
Al3+ (Aluminium ion)
(ii) Compound ions
NH4+ (Ammonium ion)
CO32- (Carbonate ion)
SO42- (Sulphate ion)
OH- (Hydroxide ion)
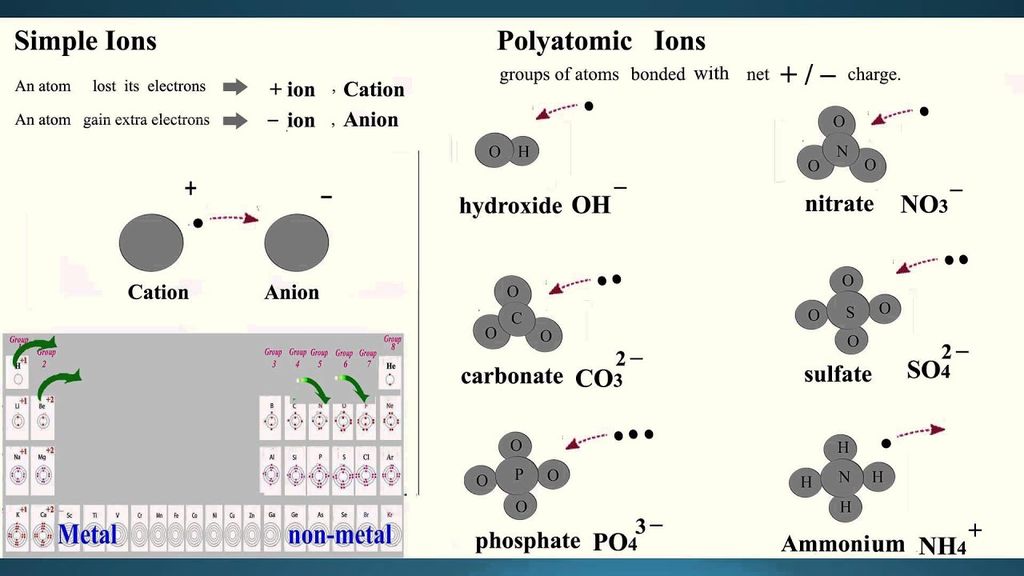
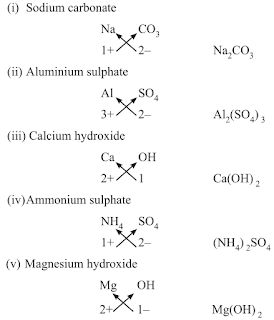
• Chemical Formulae of Ionic Compounds (Polyatomic)
Mole Concept
→ A group of 6.022×1023 particles (atoms, molecules or ions) of a substance is called a mole of
substance.
→ 1 mole of atoms = 6.022×1023 atoms
→ 1 mole of molecules = 6.022 × 1023 molecules
Example, 1 mole of oxygen = 6.022×1023 oxygen atoms
Note: 6.022×1023 is Avogadro Number (L).
→1 mole of atoms of an element has a mass equal to gram atomic mass of
the element.
Molar Mass
→ The molar mass of a substance is the mass of 1 mole of that substance.
→ It is equal to the 6.022×1023 atoms of that element/substance.
Examples:
(a) Atomic mass of hydrogen (H) is 1 u. Its molar mass is 1 g/mol.
(b) Atomic mass of nitrogen is 14 u. So, molar mass of nitrogen (N) is 14 g/mol.
(c) Molar mass of S8 = Mass of S×8 = 32×8 = 256 g/mol
(d) Molar mass of HCl = Mass of H + Mass of Cl = 1 = 35.5 = 36.5 g/mol
Important Formulae -
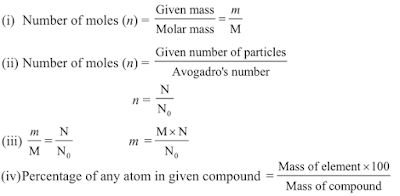

 Science Made Easy
Science Made Easy
