- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ENTHALPIES FOR DIFFERENT TYPES OF REACTIONS
• Standard Enthalpy of Formation
Enthalpy of formation is defined as the change in enthalpy in the formation of 1 mole of a substance from its constituting elements under standard conditions of temperature at 298K and 1 atm pressure.
ΔHrº (298.15 K) and ΔGrº (298.15 K)
ΔGrº (T)
ΔHrº (T) and Kp (298.15 K)
Kp (T1) and Kp (T2) where T1, T2 ≠ T
ΔHrº (T) and ΔGrº (298.15 K)
Enthalpy of Combustion: It is defined as the heat energy or change in enthalpy that accompanies the combustion of 1 mole of a substance in excess of air or oxygen.
CO(g) + ½ O2(g) ![]() CO2(g) ΔHº = -283 kJ
CO2(g) ΔHº = -283 kJ
• Thermochemical Equation
A balanced chemical equation together with the value of ΔrH and the physical state of reactants and products is known as thermochemical equation.
Thermochemical equations
- A typical chemical equation is
![]() SO2
SO2
- It is called a thermochemical equation when we add information about ΔH…
![]() SO2 ΔH = -296.9 kJ
SO2 ΔH = -296.9 kJ
- If we change the equation, then the ΔH also changes…
![]() S + O2 ΔH = +296.9 kJ
S + O2 ΔH = +296.9 kJ
- If the reaction is reversed the sign is reversed
- Also, if numbers in the equation change, so will the amount of energy produced/absorbed:
![]() 2SO2 ΔH = -593.8 kJ
2SO2 ΔH = -593.8 kJ
Conventions regarding thermochemical equations
1. The coefficients in a balanced thermochemical equation refer to the number of moles of reactants and products involved in the reaction.
If the coefficients of the chemical equation are multiplied by some factor, the enthalpy change must also be multiplied by the same factor (ΔH is an extensive property).
H2(g) + ½ O2(g) ![]() H2O(l) ΔH = -286 kJ
H2O(l) ΔH = -286 kJ
Multiply by a factor of two:
2H2(g) + O2(g) ![]() 2H2O(l) ΔH = 2 x (-286kJ) = -572kJ
2H2O(l) ΔH = 2 x (-286kJ) = -572kJ
Multiply by a factor of one-half:
½ H2(g) + ¼ O2(g) ![]() ½ H2O(l) ΔH = ½ x (-286kJ) = -134kJ
½ H2O(l) ΔH = ½ x (-286kJ) = -134kJ
• Hess’s Law of Constant Heat Summation
The total amount of heat evolved or absorbed in a reaction is same whether the reaction takes place in one step or in number of steps.
The law stats that the change in enthalpy for a reaction is the same whether the reaction takes place in one or a series of step. The Hess’s law can also be states as the enthalpy change for a chemical reaction is the same regardless of the path by which the reaction occurs.
For example, consider following two paths for the preparation of methylene chloride
Path I:
CH4 (g) + 2 Cl2(g) ![]() CH2Cl2(g) + 2HCl(g) ΔH1º = - 202.3kJ
CH2Cl2(g) + 2HCl(g) ΔH1º = - 202.3kJ
Path II:
CH4 (g) + Cl2(g) ![]() CH3Cl2(g) + HCl(g) ΔH2º = - 98.3kJ
CH3Cl2(g) + HCl(g) ΔH2º = - 98.3kJ
CH3Cl(g) + Cl2(g) ![]() CH2Cl2(g) + HCl(g) ΔH3º = - 104.0kJ
CH2Cl2(g) + HCl(g) ΔH3º = - 104.0kJ
Adding two steps
CH4(g) + 2Cl2(g) ![]() CH2Cl2(g) + 2HCl(g) ΔHº = - 202.3kJ
CH2Cl2(g) + 2HCl(g) ΔHº = - 202.3kJ
Thus whether we follow path 1 or path 2 the enthalpy change of the reaction is same.
ΔH1º = ΔH2º + ΔH3º = - 202.3kJ
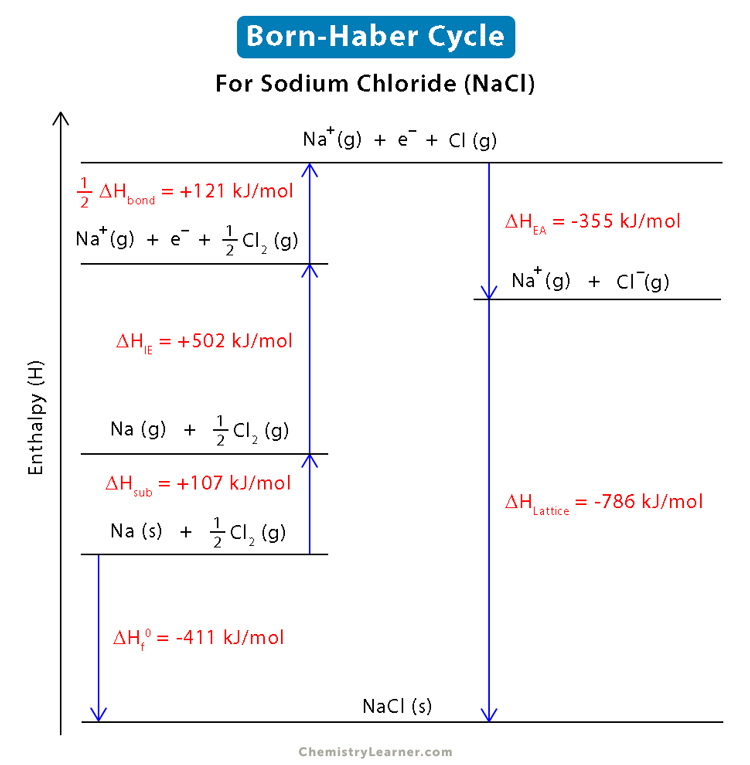
• Born-Haber Cycle
It is not possible to determine the Lattice enthalpy of ionic compound by direct experiment. Thus, it can be calculated by following steps. The diagrams which show these steps is known as Born-Haber Cycle.

 Ritan Sheth
Ritan Sheth
