- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Separation of the components of mixtures
Different methods of separation are used to get from mixture.
Heterogeneous mixtures can be separated into their respective constituents by simple physical
methods like handpicking, sieving, filtration etc.
Obtaining coloured components from blue/black ink
Process of evaporation is used to obtain coloured components from blue/black ink. The process of
evaporation is used to separate a substance which is dissolved in water.
• It is based on the fact that liquid vaporises easily than the solid.
• Helps in separating volatile substances from non-volatile substances.
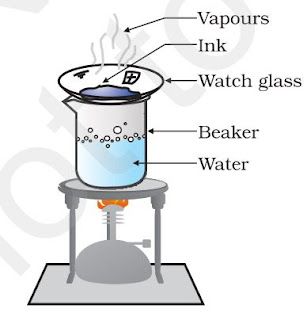
Steps of obtaining coloured components from blue/black ink:
• Fill half a beaker with water.
• Put a watch glass on the mouth of the beaker.
• Put few drops of ink on the watch glass.
• Now start heating the beaker. We do not want to heat the ink directly. You will see that evaporation
is taking place from the watch glass.
• Continue heating as the evaporation goes on and stop heating when you do not see any further
change on the watch glass.
Separation of cream from milk
• The process of centrifugation is used to separate the cream from milk. It is a method of separating
the suspended particles of substance from a liquid.
• This process is carried out by the machine called centrifuge.
• Sometimes, the solid particles in a liquid are very small and pass through a filter paper. For that
particles the filtration technique cannot be used.
• The mixture is rotated rapidly so that the heavier particles in the mixtures settle down to the
bottom.
• The basic principle of centrifugation is that the denser particles are forced to the bottom and
liquid being lighter remains at the top.
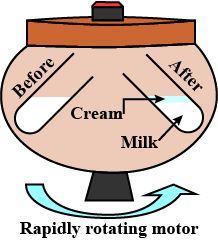
Steps of separating cream from milk:
• Take some full-cream milk in a test tube.
• Centrifuge it by using a centrifuging machine for two minutes.
Application of centrifugation:
• Used in diagnostic laboratories for blood and urine tests.
• Used in dairies and home to separate butter from cream.
• Used in washing machines to squeeze out water from wet clothes.
Separating two immiscible liquids
• The separation of separating two immiscible liquid is carried out by the use of funnel.
• The basic principle involve is the difference between the densities of two liquids form two separate layers.
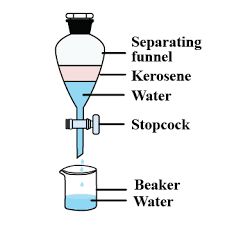
Steps of separating kerosene oil and water:
• Pour the mixture of kerosene oil and water in a separating funnel.
• Let it stand undisturbed for sometime so that separate layers of oil and water are formed.
• Open the stopcock of the separating funnel and pour out the lower layer of water carefully.
• Close the stopcock of the separating funnel as the oil reaches the stop-cock.
Application of funnel:
• To separate mixture of oil and water.
• In the extraction of iron from its ore, the lighter slag is removed from the top by this method to
leave the molten iron at the bottom in the furnace.
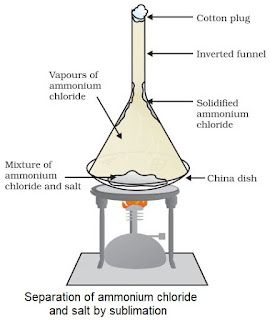
Sublimation
• This process is used to separate mixtures that contain a sublimable volatile component from
non-sublimable impurity.
• Sublimation is process where a substance directly changes from solid to gaseous state on heating.
• Ammonium chloride, camphor, naphthalene and anthracene are some examples which can be
sublime.
Chromatography
• Used to separate those solutes which dissolve in the same solvent.
• Used for sepration of colours.
• The colours which are more soluble in water rises faster.
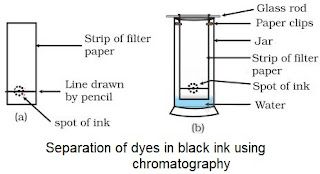
Applications
To separate
• colours in a dye
• pigments from natural colours
• drugs from blood.
Distillation
• Used for separation of components of a mixture containing two miscible liquids that boil with the
decomposition and have sufficient difference in their boiling points.
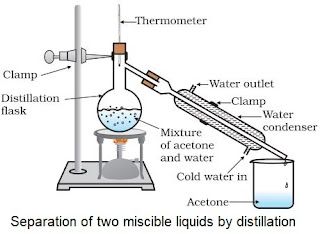
• Mixture of acetone and water is separated by this method.
Fractional distillation
• Fractional distillation is used to separate a mixture of two or more miscible liquids for which the
difference in boiling points is less than 25 K.
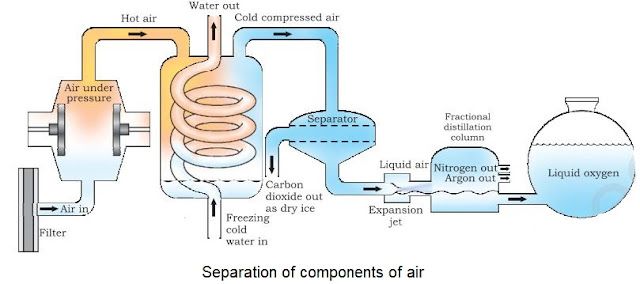
• Air is a homogeneous mixture and can be separated into its components by fractional distillation
Below is diagram which shows the steps of separation of air:
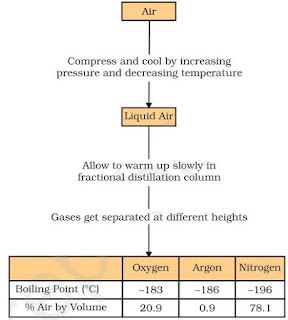
• The air is compressed by increasing the pressure and is then cooled by decreasing the temperature to get liquid air.
• The liquid air is warm-up slowly in a fractional distillation column, where gases get separated
different heights depending upon their boiling points.
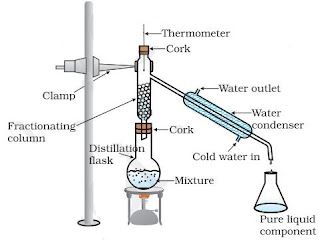
• It used to separate a gas from the air.
Crystallization
• Used to remove impurities from solid and purify it.
• It separates a pure solid from mixture in the form of crystals.
• This process is used in purification of salt from sea water, separation of crystals of alum from
impure samples.
• It is better method than evaporation because:
(i) Solids decompose or some, like sugar, may get charred on heating to dryness.
(ii) Some impurities may remain dissolved in the solution even after filtration. On evaporation the
contaminate the solid.

 Science Made Easy
Science Made Easy
