- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Evaporation
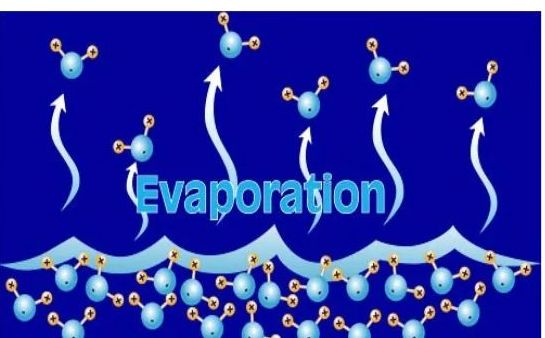
→ The process of conversion of a substance from the liquid state to the gaseous state at any temperature below its boiling point is called evaporation or vaporisation.
→ Evaporation is a surface phenomenon.
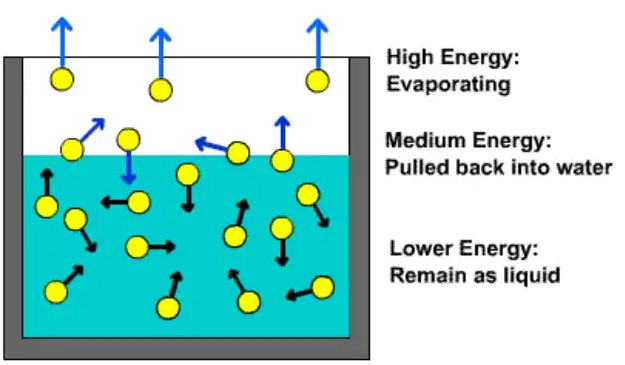
• Factors affecting the rate of evaporation
→ Surface Area – The rate of evaporation increases on increasing the surface area of the liquid.
→ Temperature - The rate of evaporation increases with an increase in temperature.
→ Humidity - Decrease in the humidity increases the rate of evaporation.
→ Wind Speed - An increase in the wind speed increases the rate of evaporation.
• Evaporation causes cooling
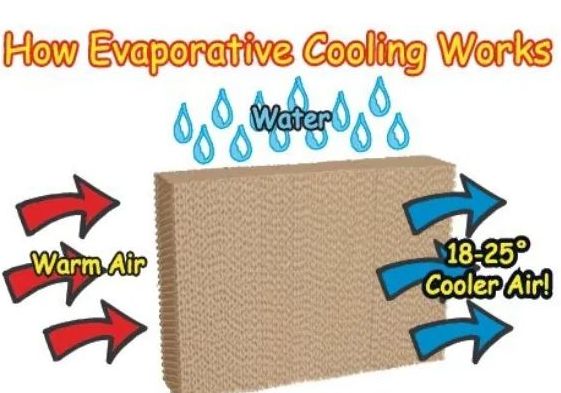
→ The particles of liquid absorb energy from the surrounding to regain the energy lost during
evaporation. This absorption of energy from the surroundings make the surroundings cold.
→ Lately, scientists are talking about five states of matter or five phases of matter. These are-so
liquids, gases, plasmas and the Bose–Einstein condensate.

 Science Made Easy
Science Made Easy
