- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Atoms
• Atoms are building blocks of all matter.
• According to modern atomic theory, an atom is the smallest particle of an element which taking
part in chemical reaction.
• Atoms are very small and which can’t be seen even through very powerful microscope.
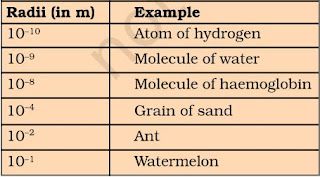
• Atomic radius is measured in nanometres. Nanometres = 10-9 m.
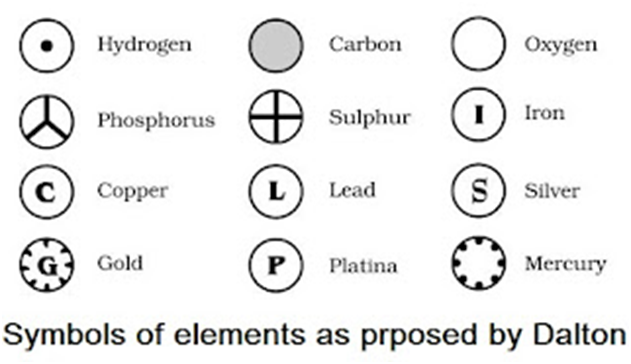
• Modern day symbols of Elements
→ Dalton was the first scientist to use the symbols for elements.
→ Berzilius suggested that the symbols of elements should be made from one or two letters of
name of the element.
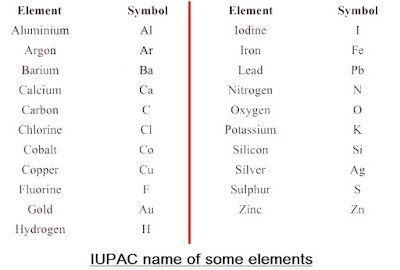
→ The name copper was taken from Cyprus, a place from where it was found for first time.
→ Now, IUPAC (International Union of Pure and Applied Chemistry) approves names of element
→ The first letter of a symbol is always written as a capital letter (uppercase) and the second letter.
as a small letter (lowercase). For example: hydrogen (H), aluminium (Al), cobalt (Co).
→ Some other symbols have been taken from the names of elements in Latin, German or Greek.For
example: Fe from its Latin name ferrum, sodium is Na from natrium, potassium is K from kalium.
• Atomic Mass
→ Dalton’s atomic theory proposed the idea of atomic mass which explained the law of constant
proportions so well.
→ The mass of an atom of an element is called its atomic mass.
→ In 1961, IUPAC have accepted ‘atomic mass unit’ (u) to express atomic and molecular mass of
elements and compounds.
→ The atomic mass unit is defined as the quantity of mass equal to 1/12 of mass of an atom of
carbon-12.
1 amu or u = 1/12 × Mass of an atom of C -12
1 u = 1.66 × 10-27 kg

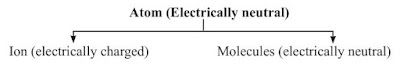
• Atom existence
→ Atoms of most of the elements are very reactive and does not exist in free state.
→ Only the atoms of noble gases (such as He, Ne, Ar, Kr, Xe and Rn) are chemically unreactive and
can exist in the free state as single atom.
→ Atoms of all other elements combine together to form molecules or ions.

 Vaishnav Publication
Vaishnav Publication
 ACERISE INDIA
ACERISE INDIA
