Solutions, Henry's law
- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
SOLUTIONS
When two or more than two substances are mixed with each other but do not react with each other it is called a solution.
Solutions are of two types
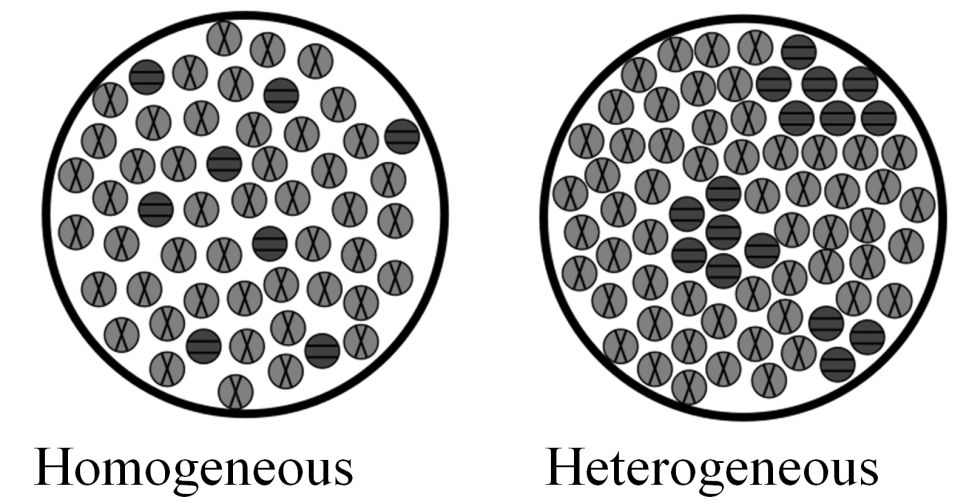
Homogeneous Solutions are mixtures of two or more than two components whose composition and properties are uniform throughout the mixture. Heterogeneous mixture don’t have uniform composition and properties
BINARY SOLUTIONS
Binary solution is a solution which contains only two components which are called solute and solvent
Solvent: The component whose mole fraction is greater than other
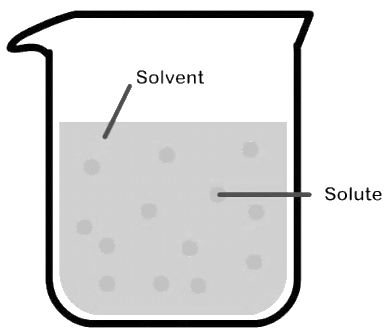
Solute: The component whose mole fraction is smaller than solvent
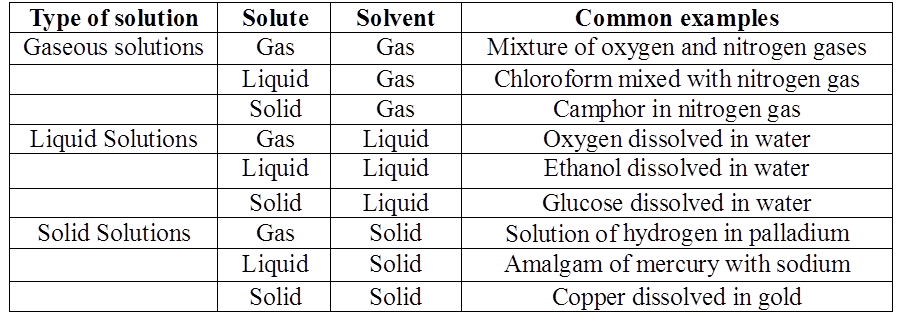
TYPES OF BINARY SOLUTIONS
CONCENTRATION OF BINARY SOLUTIONS
If we take a binary solution consisting of A & B.
- Mass percentage :
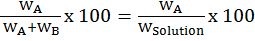
- Volume percentage :
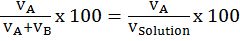
- Mass/volume percentage =
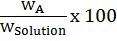
- Parts per million (ppm) =

Solution
- Mole fraction: moles of A in one mole of solution
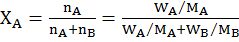
- Molality: mole of A in one kg of B

- Molarity: moles of A in one litre of solution (A + B)
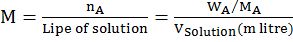
- Normality: gm eqn. of A per lite of solution
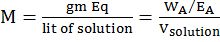
SOLUTION OF GASES IN LIQUIDS
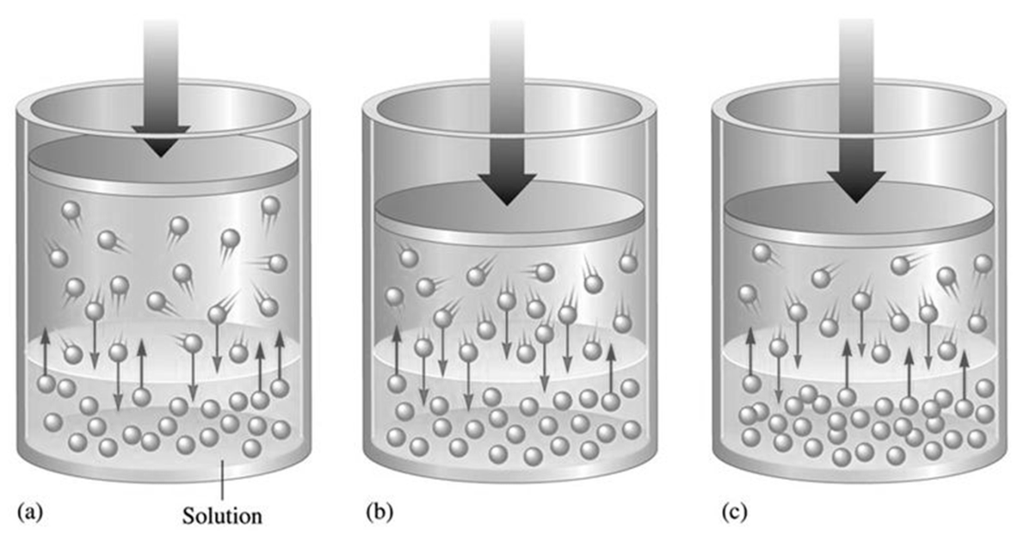
HENRY’S LAW
The mole fraction of volatile solute is proportional to the vapor pressure of the solute.
P = KHX
KH= Henry‘s Law constant, X = mole fractions.
Increasing the partial pressure of a gas over a liquid increases the amount of gas dissolved in the liquid
KH depends on temperature
Henry’s law finds several applications in industry
To increase the solubility of CO2 in soft drinks and soda water, the bottle is sealed under high pressure
Bends: Scuba divers while breathing air at high pressure underwater increases the solubility of atmospheric gases in blood. When the divers come towards surface, the pressure gradually decreases. This releases the dissolved gases and leads to the formation of bubbles of nitrogen in the blood. This blocks capillaries and creates a medical condition known as bends, which are painful and dangerous to life
Anoxia: at high altitudes due to low pressure the solubility of oxygen in blood decreases.
Effect of temperature, pressure and different gases
Temperature: Increase in temperature leads to less solubility of gases in liquid.
This can be explained by kinetic theory as well as thermodynamics
Types of Gases: Heavier gases have more solubility. At same temperature and pressure as compared to lighter gases. (Value of ‘a’)
Pressure: Increase in pressure leads to greater solubility of gases in liquids.
SOLUTIONS LIQUID IN LIQUID
In this Section, we shall discuss the solutions of liquids and solids in a liquid. Such solutions may contain one or more volatile components. Ex: water-alcohol
- Solubility: Solubility of a substance is defined as the maximum amount of substance that can be dissolved in a specified amount of solvent to a given temperature
- Saturated solution : A solution in which no more solute can be dissolved at the same temperature and pressure is called a saturated solution
- Effect of temperature: mass %, mole fraction and molality do not change with temperature whereas Molarity changes with temperature because volume of solution (liquid) changes with temperature.
- Effect of dilution;-Moles, milli-moles, equivalent, milli-equivalent of solute does not change on dilution, but Molarity , molality and mole fraction change with dilution
- Like dissolves like: Polar solutes dissolve in polar solvents and non-polar solutes dissolves in non-polar solvents. In general, a solute dissolves in a solvent if the intermolecular attractions are similar in both.
A liquid may or may not be solute in another liquid Depending upon the relative solubility of a liquid in another the following three cases are possible:

Miscible liquid form three types of solution, which can be ideal or non ideal solutions.
When solute and solvent both are Volatile
Volatile: When the vapour pressure of substance is low. Ex. Ethyl alcohol, hexane etc.
Non Volatile: When the vapour pressure of substance is high. All solids are non-volatile. Some liquids like Glycerol etc. are non-volatile.
Vapour pressure
- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
VAPOUR PRESSURE
Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium.
1) Imagine a closed box of several liters in size. It has rigid walls and is totally empty of all substances.
2) Now, I inject some liquid into the box, but the box is not full of liquid. What will happen to the liquid?
3) That's right. Some, maybe all, of the liquid will evaporate into gas, filling the empty space. Now if all the liquid evaporates, we just have a box of gas. That's not what we want. So let's suppose that only some of the liquid evaporated and that there are both the liquid state and the gas state present in the box.
The gas that is above the liquid is called its vapor and it creates a pressure called vapor pressure.
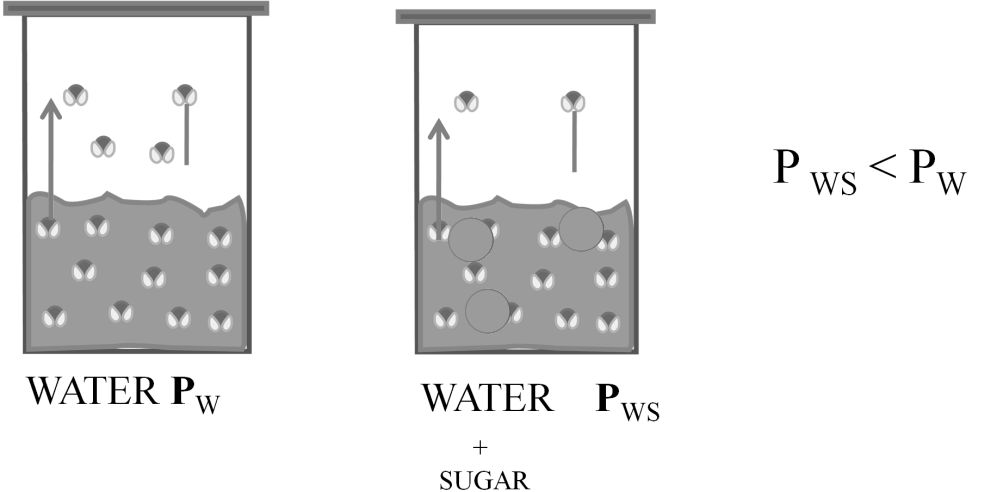
RAOULT’S LAW
When two liquids (A&B) are mixed.
Partial pressure of A = PPA = XA P°A
Partial pressure of B = PPB = XB P°B
P°A = vapour of pressure of pure A
P°B = vapour Pressure Of pure B
Total pressure P = PPA + PPB ® Dalton’s law
P = P°A XA + P°B XB ® Raoult’s law
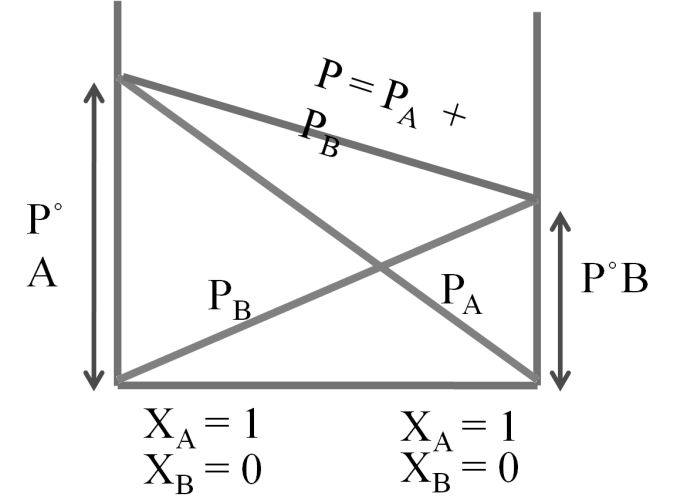
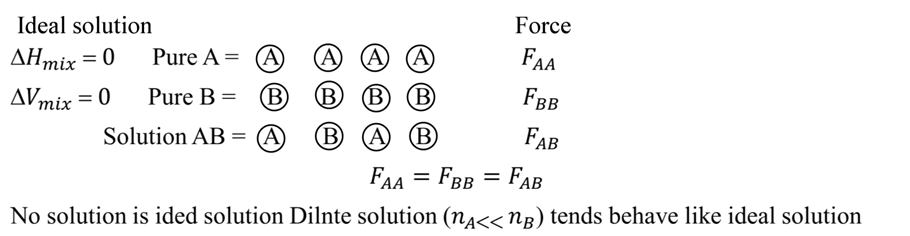
Ideal and non-ideal solutions

Non ideal solutions
For a non-ideal solution
(1) DHmix ¹ 0 (It may be less or more)
(2) DVmix ¹ 0 (It may be less or more)
(3) Does not follow Raoult’s law.
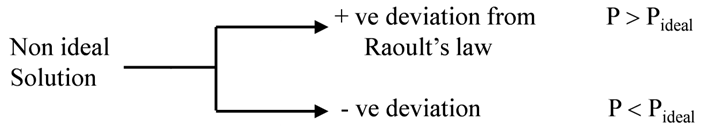
Non ideal solutions
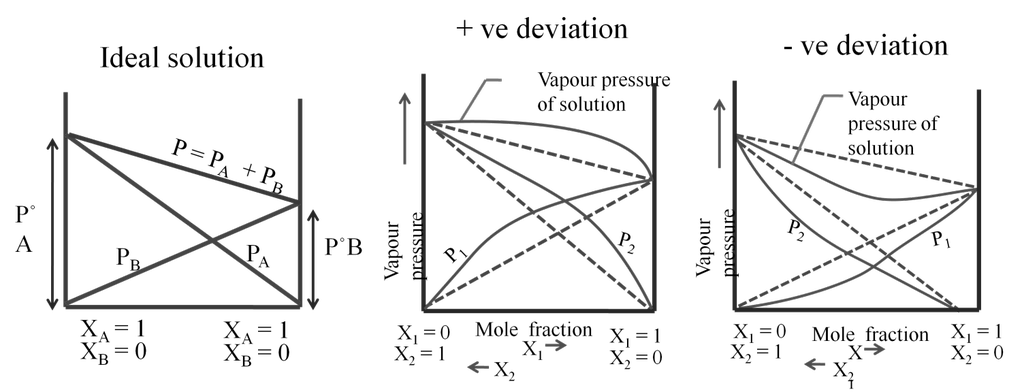
Non ideal solutions

Azeotropes are binary solutions (liquid mixtures) having the same composition in liquid and vapour phase and it is not possible to separate the components of an azeotrope by fractional distillation.
Types of azeotropes:
(a) Minimum boiling azeotrope: The non- ideal solutions showing large positive deviation form minimum boiling azeotrope at a specific composition. Example; 95% ethanol and 5% water (by volume) Ethanol = 351.3K, Water = 373 K, Azeotrope = 351.1K
(b) Maximum boiling azeotrope. The non- ideal solutions showing large negative deviation form maximum boiling azeotrope at a specific composition. Example : 68% Nitric acid and 32% water (by mass) Nitric acid = 359K , Water = 373 K, Azeotrope = 393.5K
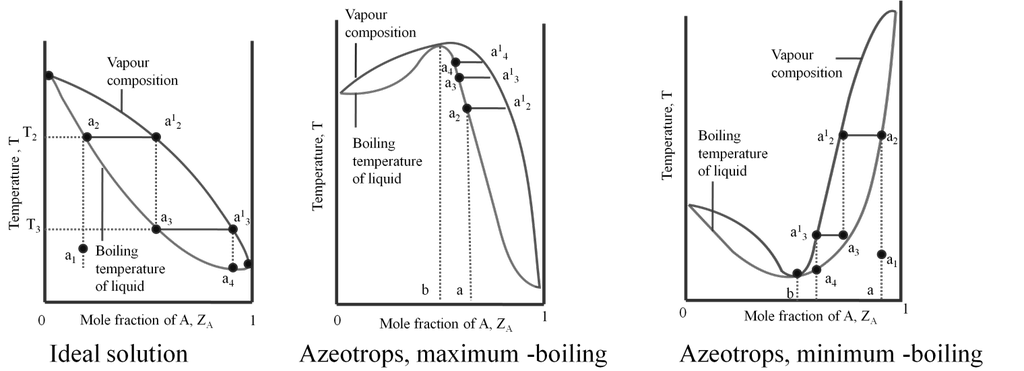
Solid in liquid solutions
- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
SOLID IN LIQUID SOLUTIONS
Solubility of solid (or Non-volatile liquids) in Liquid
Colligative Properties: The properties of solutions which depends on the no. of solute particles but irrespective of nature of solute particles.
There are four Colligative properties:
a. Relative lowering of vapour pressure
b. Elevation of boiling point
c. Depression of freezing point
d. Osmotic pressure
a. Relative lowering of vapour pressure
P = poA XA + PoB XB → Raoult’s law
Dilute solution is where nA < < nB
Or XA < < < XB
Non volable solute is where ![]()
![]()
![]()
![]()
![]()
b. Elevation of boiling point
Boiling point is the temperature at which the vapour pressure of liquid equals the atmospheric pressure.
Elevation in boiling point (∆Tb) is the difference in boiling point of pure liquid and boiling of solution of this liquid with a non-volatile solute.
![]()
Solutions
As can be clean seen from graph
![]()
![]()
![]()
![]()
![]()
![]()
Kb = Boiling point elevation constant of modal elevation constant (Ebullioscopic) constant
c. Depression of freezing point:
Freezing point: Temperature at which the vapour pressure of the substance in its liquid phase is equal to its vapour pressure in its solid phase
The lowering of vapour pressure of solution causes a lowering of freezing point compared to that of pure solvent. The difference in freezing point of the pure solvent and solution is called depression in freezing point
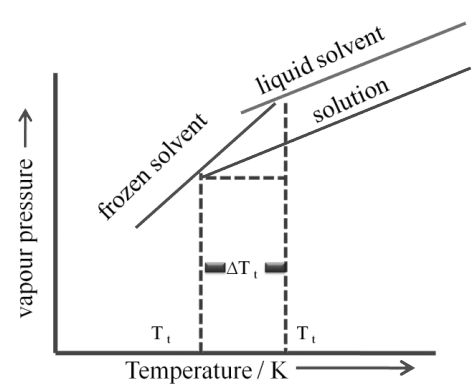
Depression in freezing point
Similar to elevation in boiling point
∆Tf α ∆P
α XA P˚ solvent
α molality
![]()
Kf = depression in freezing point constant or molal depression constant or cryoscopic constant.
Osmotic pressure
- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
d. Osmotic pressure
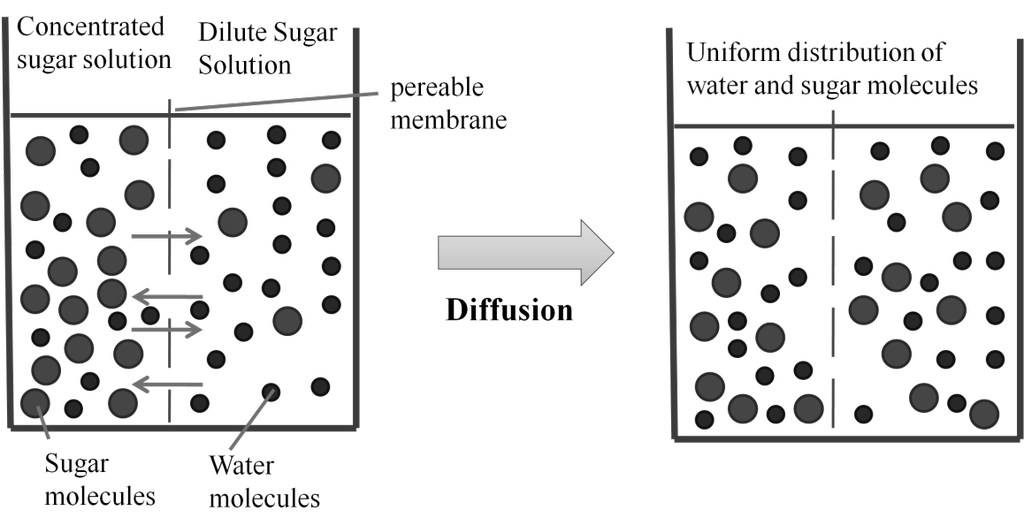
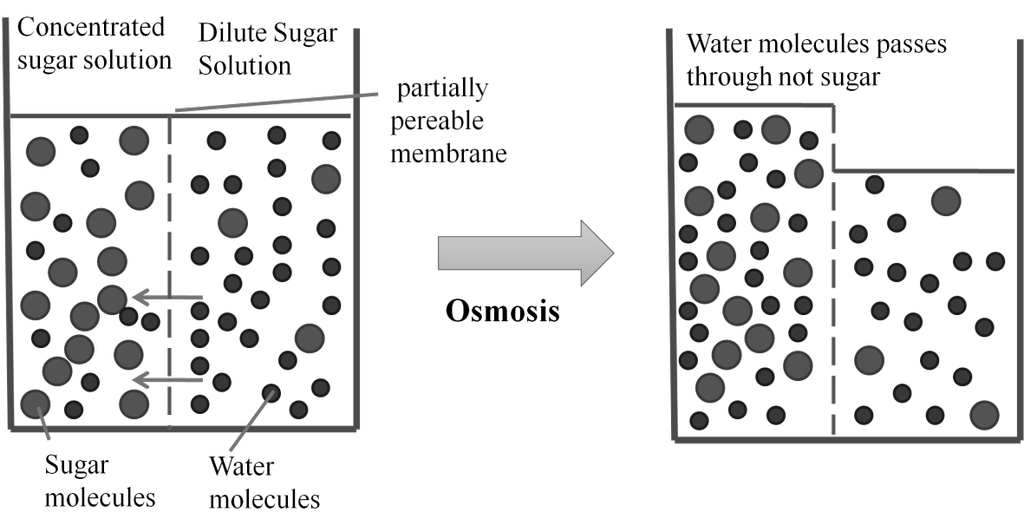
Osmosis: The process of flow of solvent molecules from pure solvent to the solution through semi permeable membrane.
Osmotic pressure (p) : Minimum pressure that must be applied to the solution to prevent the flow of solvent into the solution through a semipermeable membrane
p(Osmotic pressure) = nVRTi=wmVRTi
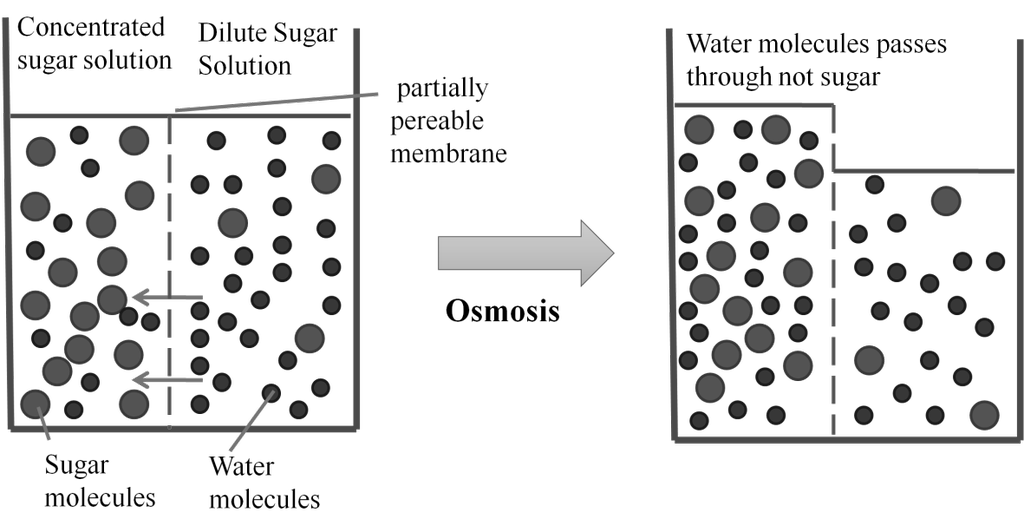
Isotonic solution: Two solutions having same osmotic pressure at a given temperature are called isotonic solution p1 = p2
Hypertonic solutions: If a solution has more osmotic pressure than other solution it is called hypertonic solution.
Hypotonic solution: If a solution has less osmotic pressure than other solution it is called hypotonic solution
Reverse osmosis: When a pressure larger than osmotic pressure is applied to the solution side, the pure solvent flows out of solution to solvent side through semi permeable membrane this process is called reverse osmosis Use: Reverse osmosis is used to desalination of sea water (into drinking water)
Application of Colligative property: By measuring a colligative property of a solution molar mass of the non-volatile solute can be calculated Conditions for getting accurate value of molar mass:
- Solute must be non-volatile
- Solution must be dilute
- Solute particles must not undergo any association or dissociation in the solution
Abnormal molar mass and Van’t Hoff factor (i): When the non-volatile solute particles undergo association (Eg: acetic acid in benzene) or dissociation (Eg: electrolytes like KCl, NaCl in water), the abnormal molar mass is observed.
NaCl No+ + Cl-
1 mole 0 0 total moles = 0
1- α α α total moles= 1+α
α = degree of dissociation
Actual moles are (1 + α) not (1)
![]()
![]()
![]()
1. For solutions undergoing dissociation
i >1 { Exp coll .prop > normal cell. Property}
{exp. Mol. wt < normal mol.wt }
2. For solution undergoing association
i < 1 { exp. Coll. Property < normal. Coll. property}
{ exp. Mol. Wt > normal .mol .wt}
![]()
![]()
Association of solute
CH3COOH Anociation in benzene to form dimer
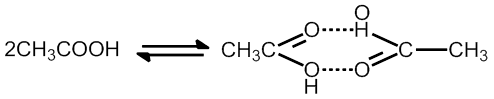
Note CH3COOh dissociate in water
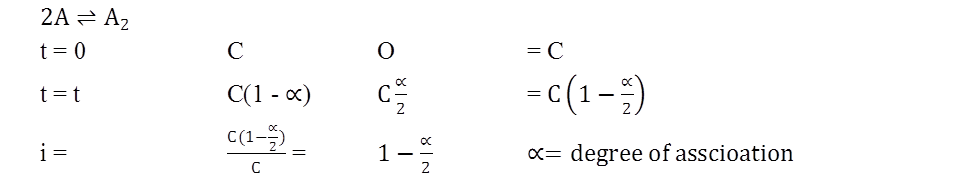
General formula (both for anociation an well an dissociation)
![]()
y = no. of particle from parhole.
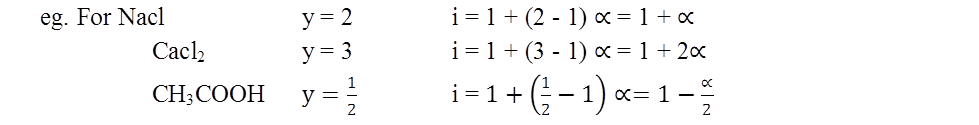
Important Formulae:
- p(osmotic pressure) =
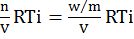
- Isotonic solution p1 = p2
- Vapour pressure variation with temperature 2.303 log10
 =
= 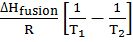
- Raoult’s Law For liquid-liquid systems: P=PPA+PPB+…
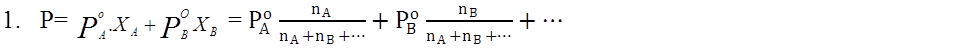
Relative lowering of vapour pressure:
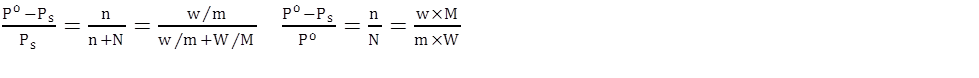 [for dilute solutions]
[for dilute solutions]
- Elevation in boiling point. ∆Tb=Kb×molality xi=1000 KbwmWi
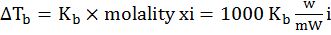
- Depression in freezing point: ∆Tf=Kf×molality xi=1000 KfwmWi
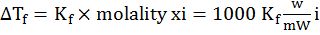
 and
and 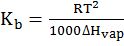
- For solutes undergoing dissolution AxByà xAy++ yBx- i>1. Experimental colligative property > normal colligative property and Experimental molecular weight < normal molecular weight
- For solutes undergoing association A +A +..nà An i<1 Experimental colligative property < normal colligative property and Experimental molecular weight > normal molecular weight
- Vant Hoff Coefficient i = 1 + (y-1)a a=degree of dissociation or association and y = no of particles from one particle

![]()

 Kaysons Publication
Kaysons Publication
