- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
RUTHERFORD MODEL
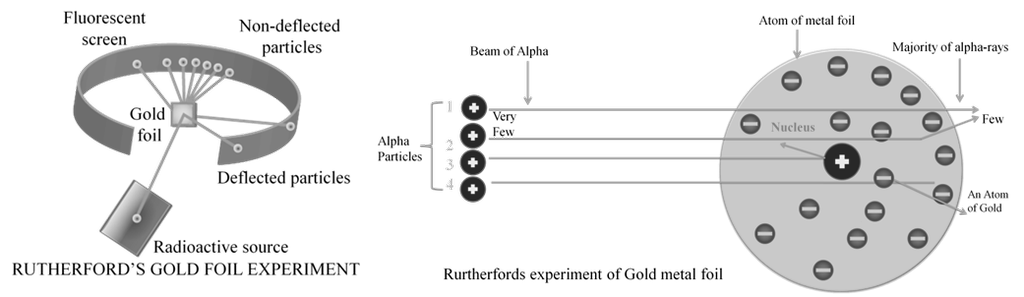
Observations from a - particles scattering experiment by Rutherford:
a. Most of the a - particles (nearly 99 %) passed through gold foil undeflected
b. A small fraction of a- particles got deflected through small angles
c. Very few a - particles did not pass through foil but suffered large deflection nearly 180O
Inferences Rutherford drew from - particles scattering experiment:
a. Since most of the a -particles passed through foil undeflected, it means most of the space in atom is empty
b. Since some of the a -particles are deflected to certain angles; it means that there is positively mass present in atom
c. Since only some of the a -particles suffered large deflections; the positively charged mass must be occupying very small space
d. Strong deflections or even bouncing back of a -particles from metal foil were due to direct collision with positively charged mass in atom
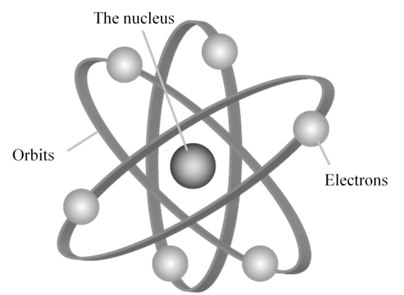
As per Rutherford the size of nucleus is 104-105 times smaller than atom. Mardsen & Rutherford calculated density of nucleus.
Volume of atom / Volume of nucleus = 1015
Volume of earth approximately 1.08x1021 cubic meters
If earth was an atom then volume of nucleus = 1.08x1021/1015 = 1.08x106
That is ball of radius = 44m
Rutherford and Marsden gave an equation to find radius of nucleus of any atom.
R = RO A1/3
Ro = 1.33x 10-13 cm
A = no. of nucleons (Neutrons + protons)
Example calculate the density of nucleus of oxygen atom.
![]()
![]()
=1.685 x 1014 gm. /cm3 = 1.685 x 1017 kg/m3
Mass of moon = 7.34767309 × 1022 kilograms
If moon is all made of nucleus it will become a ball of radius 32m. Slightly bigger than basketball court!!!!!!!
Drawback of Rutherford model
- According to Rutherford’s model of atom, electrons which are negatively charged particles revolve around the nucleus in fixed orbits. Thus, the electrons undergo acceleration. According to electromagnetic theory of Maxwell, a charged particle undergoing acceleration should emit electromagnetic radiation. Thus, an electron in an orbit should emit radiation. Thus, the orbit should shrink. But this does not happen.
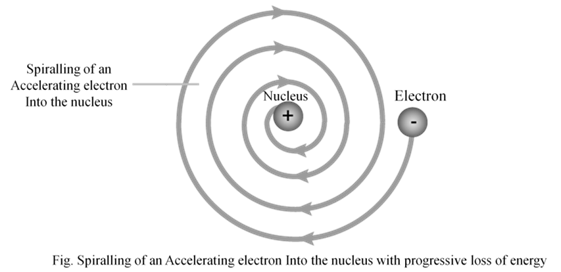
2. The model does not give any information about how electrons are distributed around nucleus and what are energies of these electrons
Atomic number (Z): It is equal to the number of protons in an atom. It is also equal to the number of electrons in a neutral atom.
Mass number (A): It is equal to the sum of protons and neutrons.
Isotopes: These are the atoms of the same element having the same atomic number but different mass number. Ex 6C12 and 6C14
Isobars: Isobars are the atoms of different elements having the same mass number but different atomic number. 18Ar40 and 20Ca40
Isoelectronic species: These are those species which have the same number of electrons. Ex. N2 and CO
Isotones: Two nuclides are called isotones if they have same neutron number N, but different proton numbers Z. For example, Boron- 12 and Carbon-13 nuclei, but both contain 7 neutrons
ELECTROMAGNETIC WAVES
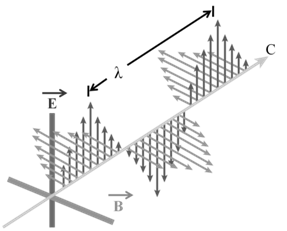
The radiations which are associated with electrical and magnetic fields are called electromagnetic radiations. When an electrically charged particle moves under acceleration, alternating electrical and magnetic fields are produced and transmitted. These fields are transmitted in the form of waves. These waves are called electromagnetic waves or electromagnetic radiations.
PROPERTIES OF ELECTROMAGNETIC RADIATIONS:
a. Oscillating electric and magnetic field are produced by oscillating charged particles. These fields are perpendicular to each other and both are perpendicular to the direction of propagation of the wave.
b. They do not need a medium to travel. That means they can even travel in vacuum.
WAVE NATURE OF ELECTROMAGNETIC RADIATION
Characteristics of electromagnetic radiations:
a. Wavelength: It may be defined as the distance between two neighbouring crests or troughs of wave as shown. It is denoted by l.
b. Frequency (n): It may be defined as the number of waves which pass through a particular point in one second.
c. Velocity (v): It is defined as the distance travelled by a wave in one second. In vacuum all types of electromagnetic radiations travel with the same velocity. Its value is 3 X108 m sec-1. It is denoted by v
d. Wave number: Wave number (ν![]() ) is defined as the number of wavelengths per unit length.
) is defined as the number of wavelengths per unit length.
Relationship between velocity, frequency and wavelength Velocity = frequency x wavelength
Velocity = frequency x wavelength

THE SPECTRUM OF ELECTROMAGNETIC RADIATION
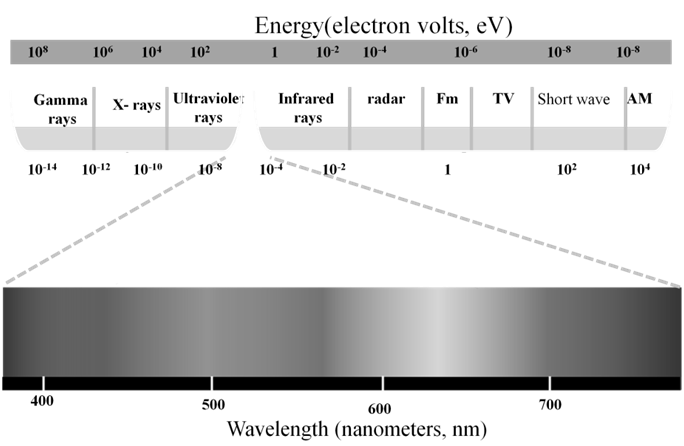
Ex.-The Vividh Bharati station of All India Radio, Delhi, broadcasts on a frequency of 1,368 kHz (kilo hertz).
Calculate the wavelength of the electromagnetic radiation emitted by transmitter. Which part of the electromagnetic spectrum does it belong to?
![]()
![]()
![]() = 219.3m
= 219.3m
This is a characteristic radio wave wavelength.
Ex.: Calculate (a) wave number and (b) frequency of yellow radiation having wavelength 5800 Å.
Solution
- Calculation of wavenumber
λ = 5800Ǻ = 5800 x 10-8 cm
= 5800 x 10-10 m
![]()
= 1.724 x 106 m-1
= 1.724 x 104 cm-1
(b) Calculation of the frequency (n)
![]()
TYPES OF SPECTRUM (CAN BE CLASSIFIED IN TWO DIFFERENT WAYS
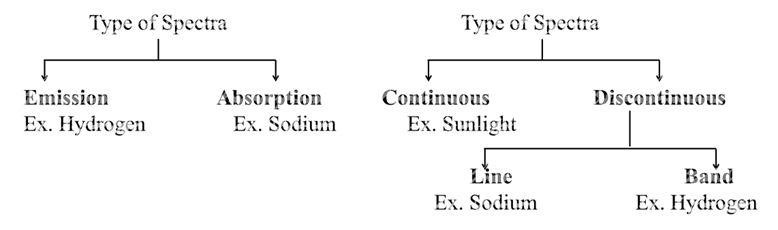
FIRST CLASSIFICATION SPECTRUM IS OF TWO TYPES: CONTINUOUS AND LINE SPECTRUM
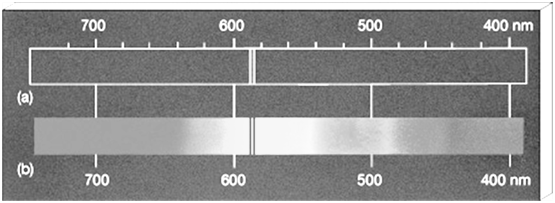
a. The spectrum which consists of all the wavelengths is called continuous spectrum.
b. A spectrum in which only specific wavelengths are present is known as a line spectrum. It has bright lines with dark spaces between them.
Electromagnetic spectrum is a continuous spectrum. It consists of a range of electromagnetic radiations arranged in the order of increasing wavelengths or decreasing frequencies. It extends from radio waves to gamma rays.
SPECTRUM IS ALSO CLASSIFIED AS EMISSION AND ABSORPTION SPECTRUM.
- Emission spectrum: A substance absorbs energy and moves to a higher energy state. The atoms, molecules or ions that have absorbed radiation are said to be excited. Since the higher energy state is unstable they return to the more stable energy state by emitting the absorbed radiation in various regions of electromagnetic spectrum. The spectrum of radiation emitted by a substance that has absorbed energy is called an emission spectrum.
- Absorption spectrum is the spectrum obtained when radiation is passed through a sample of material. The sample absorbs radiation of certain wavelengths. The wavelengths which are absorbed are missing and come as dark lines

 Kaysons Publication
Kaysons Publication
