- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Atomic Number
→ The total number of proton lying in the nucleus of any atom is called the atomic number.
→ An atomic number is the identity of an atom, changing atomic number means changing the atom.
→ Atomic number is denoted by ‘Z’.
→ Atomic number = no. of protons or a neutral atom, no. of protons and electrons are equal.
Mass Number
→ It is the sum of total number of protons and no. of neutrons lying in the nucleus of an atom.
→ It is denoted by ‘A’.
→ Mass number = no. of protons + no. of neutrons
→ Representation of an atom:
zXA
(X= symbol of an element)
Example: Calculate number of protons, electrons and neutrons for 17Cl35 or 17Cl37
Sol. Since Cl is neutral,
No. of electrons = no. of protons = 17
Mass no. of Cl = 35
No. of neutrons = 35 - 17 =18
Distribution Of Electrons In Various Shells
→ The distribution of electrons in various shells is done in accordance to ‘Bohr-Bury Scheme’.
Bohr-Bury Scheme
(i) The filling of electrons in an atom is done in accordance to ‘2n2’, where ‘n’ is the number of shell and ‘2n2 ’ represents the total number of electrons that can be accommodated in that particular shell.
→ Maximum number of electrons that can be filled in particular shell.
If n = 1, i.e., K = shell, 2n2= 2×1 2 = 2 electrons
If n = 2, i.e., L = shell, 2n2 = 2×2 2 = 8 electrons
If n = 3, i.e., M = shell, 2n2 = 2×3 2 = 18 electrons
If n = 4, i.e., N = shell, 2n2 = 2×4 2 = 32 electrons
(ii) The outermost shell can’t hold more than 8 electrons, while second last shell can’t have more than 18 electrons, even though they may have capacity to hold more electrons.
Example: ‘Ca 20 ’, the electron distribution will be :
Ca 20 = 2(K), 8(L), 8(M), 2(N)
→ But Ca 20 = 2, 8, 10 is wrong although ‘M’ shell can contain upto 18 electrons.
(iii) The outermost shell can’t hold more than 2 electrons and the penultimate shell can’t hold more than 8 electrons unless the preceding inner shell (antepenultimate shell) is filled completely obey ‘2n2 ’ rule.
(i) K(19) = 2, 8, 8, 1
(ii) Al (13) = 2, 8, 3
(iii) F (9) = 2, 7
(iv) Ne (10) = 2, 8
(v) Na (11) = 2, 8, 1
Valence Shell and Valence Electrons
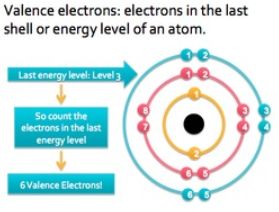
→ From Bohr-Bury sequence, we know that maximum number of electrons which can be accommodated in outermost shell is 8.
→ Every element has an urge to have 8 electrons in its outermost shell, in achieving 8 electrons atom can either gain electrons or loose electrons.
→ The number of electrons lost or gained by an element in achieving 8 electrons in its outermost shell will be called its Valency.
→ For elements like H, He, Li, Be and B, these elements lose their outermost electron to achieve 2 electrons in their outermost shell.
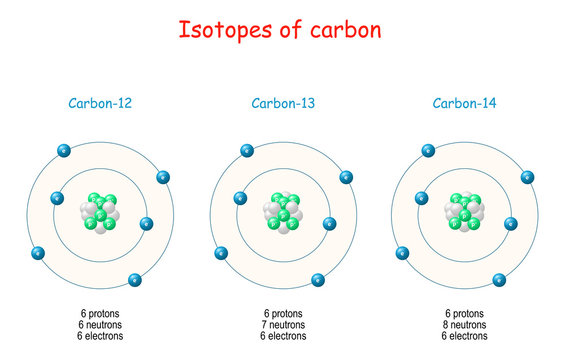
→ Isotopes are atoms of same elements having same atomic number and different mass number.
Example: Chlorine has two isotopes of mass numbers 35 and 37 respectively.
17Cl35 , 17Cl37
Uses of isotopes
(i) Uranium isotope is used as fuel in nuclear rector.
(ii) Isotope of cobalt is useful in treatment of cancer.
(iii) An isotope of iodine is used in the treatment of goitre.
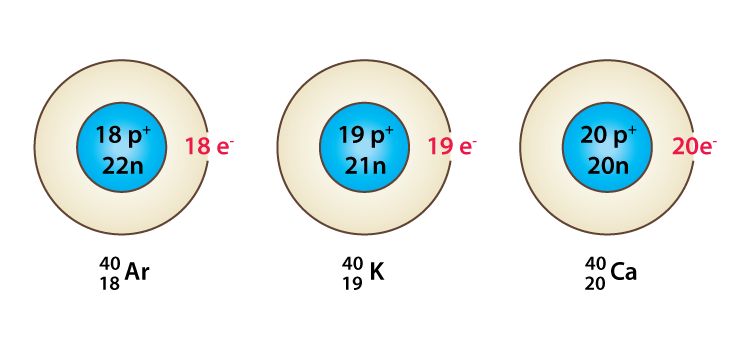
Isobars
→ Isobars are the atoms of those elements which have the same mass number but different atomic
numbers are called isobars.
→ 20Ca40 and 18Ar40 have same mass number and different atomic number. 11Na24 and 12Mg24
another examples.

 Science Made Easy
Science Made Easy
