- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Colloidal solution and its properties
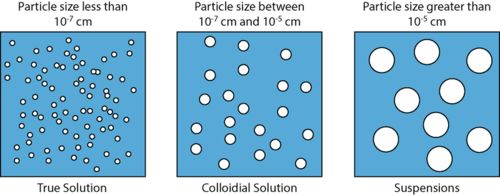
Colloid solution is heterogeneous mixture in which the size of particles lies between the true solutions and suspensions.
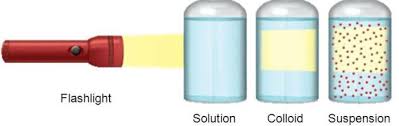
• Colloidal particles can easily scatter a beam of visible light. This phenomenon is called
Tyndall effect.
Properties of colloidal solution:
1. The particles of colloid can’t be seen by naked eyes individually.
2. It is a heterogeneous mixture and thus solute and solvent can’t be separated by filter paper.
3. Size of particles is smaller than suspensions but greater than solutions (1 nm to 100 nm).
4. It is a stable mixture. Particles do not settle down at the bottom over a period of time.
5. They do not settle down when left undisturbed which means colloid is quite stable.
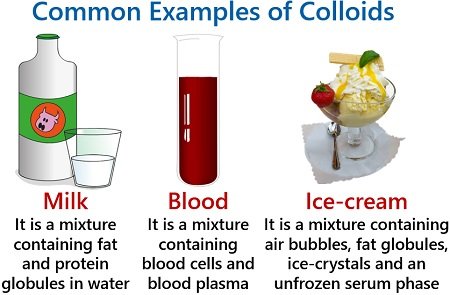
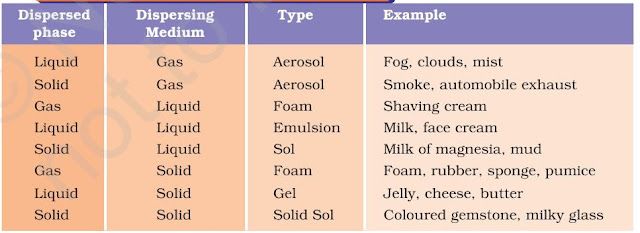
Some common examples of colloids (in the table)

 Vaishnav Publication
Vaishnav Publication
 ACERISE INDIA
ACERISE INDIA
