- Books Name
- CBSE Class 7 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 7
- Subject
- Science
2.2 ACIDS
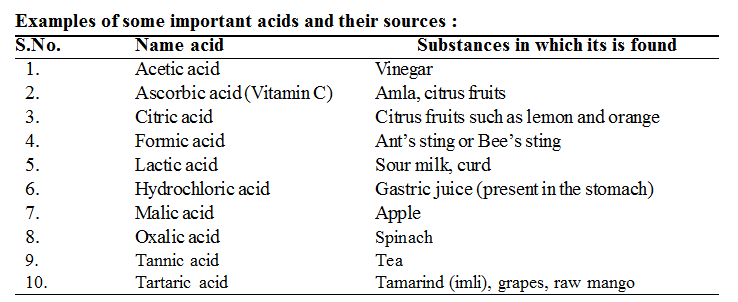
The term acid is derived from the Latin word acidus meaning sour. Lemons, oranges and grapes taste sour because they contain citric acid. Tamarind and vinegar contain tartaric acid and acetic acid respectively.
Definition :
“An acid is defined as a substance which gives H+ ions on dissolution in water.”
e.g.
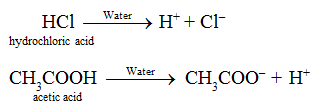
Nitric acid (HNO3), Phosphoric acid (H3PO4) , formic acid (HCOOH) etc. have one and sulphuric acid (H2SO4) has two replaceable hydrogen atom, thus they are acids.
Remember
• Vitamin C which is very important for our body is also an organic acid known as ascorbic acid.
Classification of Acids:
(a) On the basis of occurrence:
(i) Mineral acids : Acids which are obtained from the minerals present in earth’s crust are called mineral acids.
e.g. HCl, H2SO4, HNO3 etc.
(ii) Organic acids : Acids that are found in animals and plants are known as organic acids.
e.g. Lactic acid , citric acid, tartaric acid, acetic acid and formic acid.
(b) On the basis of strength:
(i) Strong Acids :
Acids, which almost completely ionise (break up into ions) in water, are called strong acids.
e.g. Hydrochloric acid (HCl), sulphuric acid (H2SO4), nitric acid (HNO3) etc.
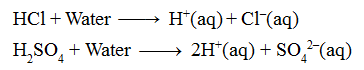
(ii) Weak Acids:
Acids, which partially ionise in water, are called weak acids.
e.g. Carbonic acid (H2CO3), phosphoric acid (H3PO4), formic acid (HCOOH), acetic acid (CH3COOH).
![]()
REMEMBER
• The sharp pain caused by the sting of ants and bees is due to formic acid, which they push into the body or spray on the skin.
• Acids like conc. H2SO4 and conc. HNO3 are corrosive in nature. They destroy organic matter like clothes, paper, wood and cause burn to human skin.
• In general mineral acids are strong while organic acids are weak
(c) On the basis of concentration :
(i) Concentrated acid :
The acid containing very less amount of water is called concentrated acid. HCl is prepared by dissolving HCl gas in water. The solution of this acid is called conc. HCl.
(ii) Dilute acid : The acid containing excess amount of water is called dilute acid. Strength can be decreased by dissolving the acid in more water. In a laboratory, we generally use either concentrated acid or it’s solution diluted to a definite strength.
Advance Learning
Dilution of acids :
It is always desirable to add acid to water, keeping the solution continuously stirred, while preparing dilute solutions of acids, specially mineral acids. We should always slowly add acid to water; otherwise, so much heat is produced during the dilution process that the container, specially that of glass, may break. The hot contents may also cause an explosion and spill on our clothes and body. This may result into serious acid burns.
(d) On the basis of basicity :
(i) Monobasic Acids :
When one molecule of an acid on complete ionisation produces one hydronium ion (H3O+) in aqueous solution, the acid is said to be a monobasic acid.
Examples of Monobasic Acids.
Some examples of monobasic acids are :
(i) Hydrochloric acid (HCl) (ii) Hydrobromic acid (HBr)
(iii) Nitric acid (HNO3) (iv) Acetic acid (CH3COOH)
(v) Formic acid (HCOOH)
(ii) Dibasic Acids :
When one molecule of an acid on complete ionisation produces two hydronium ions (H3O+) in aqueous solution, the acid is said to be a dibasic acid.
Examples of Dibasic Acids :
Some examples of dibasic acids are :
(i) Sulphuric acid (H2SO4) (ii) Sulphurous acid (H2SO3)
(iii) Carbonic acid (H2CO3) (iv) Oxalic acid [(COOH)2]
(v) Hydrofluoric acid (HF)
(iii) Tribasic Acids :
When one molecule of an acid on complete ionisation produces three hydronium ions (H3O+) in aqueous solution, the acid is said to be a tribasic acid.
An example of tribasic acids is Phosphoric acid (H3PO4).
(iv) Tetrabasic Acids :
When one molecule of an acid on complete ionisation produces four hydronium ions (H3O+) in aqueous solution, the acid is said to be a tetrabasic acid.
An example of tetrabasic acids is silicic acid (H4SiO4)
Remember
• The atmosphere of Venus is made up of thick white and yellow clouds of Oil of Vitriol (H2SO4 ).

 Grow Career Publication
Grow Career Publication
 Param Publication
Param Publication
