- Books Name
- CBSE Class 7 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 7
- Subject
- Science
(a) Neutralisation in everyday life:
(i) Indigestion : People particularly of old age suffer from acidity problems in the stomach which is caused mainly due to release of excessive gastric juices containing HCl. The acidity is neutralised by antacid tablets which contain sodium hydrogen carbonate (baking soda, NaHCO3), magnesium hydroxide (milk of magnesia, Mg(OH)2) etc.
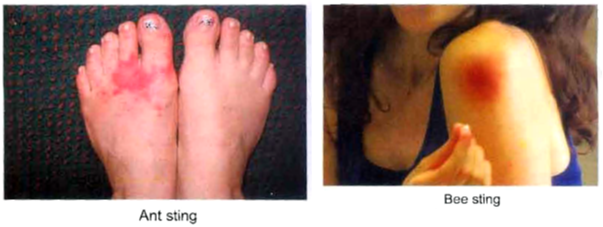
(ii) Ant and bee sting : The stings of bees and ants contain formic acid. Its corrosive and poisonous effect can be neutralised by rubbing soap which contains NaOH (an alkali) or by rubbing baking soda (NaHCO3) or by calamine solution (ZnCO3). The stings of wasps contain an alkali and its poisonous effect can be neutralised by an acid like acetic acid (present in vinegar).
(iii) Soil treatment : Farmers generally neutralize the effect of acidity in the soil caused by acid rain by adding slaked lime (Calcium hydroxide, Ca(OH)2) to the soil.
(iv) Factory wastes : The wastes of many factories contain acids. If they are allowed to flow into the water bodies, the acids will kill fish and other organisms. The factory wastes are, therefore, neutralised by adding basic substances.
POINTS TO REMEMBER
· Atom : The smallest particle of an element that takes part in a chemical reaction is an atom.
· Element : Element is the basic constituent of all matter.
· Chemical Compound : A substance whose each molecule contains two or more atoms of different elements in a fixed ratio is a chemical compound.
· Acid : The substance which contains hydrogen and produces H+ ions in aquesous solution is called Acid. Acids are sour in taste.
· Base: The substance which produces OH– ions in aqueous solution is called the chemical substances which are bitter in taste and soapy base touch.
· Alkalis : Bases which dissolves in water are called alkalis.
· Neutralisation : The reaction between an acid and a base is known as neutralisation.
· Antacid : It is a medicine that neutralize acid formed in the stomach.
· Litmus, turmeric and china rose petal are naturally occurring indicators, while methyl orange and phenolphthalein are prepared in laboratories.
· On the basis of chemical nature, all chemical substances are broadly classified as acidic, basic and neutral substances.
· Acid Rain : When pollutent like sulphur dioxide and nitrogen oxides dissolve in rain water, it forms an acid. The rain of that acid is called acid rain.
Neutralization in everyday life
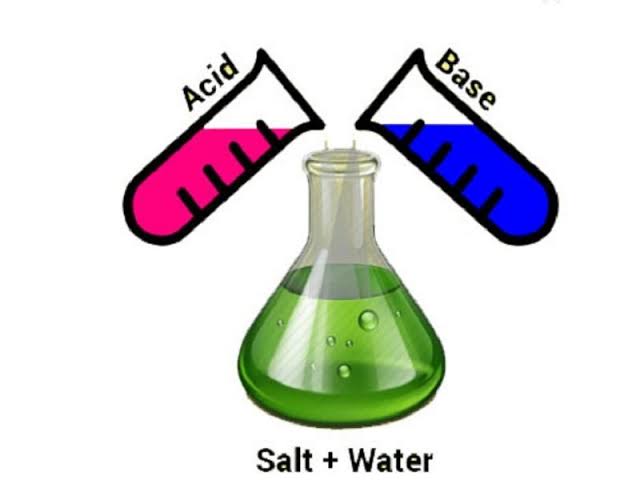
The reaction between an acid and a base is called neutralisation. In everyday life, it is employed in different applications. For example, it is used in the neutralisation of stomach acidity, in the prevention of tooth decay, neutralising the soil, in the treatment of ant’s bites, etc

 Param Publication
Param Publication
 Grow Career Publication
Grow Career Publication
