- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ANOMALOUS BEHAVIOUR OF BERYLLIUM
The anomalous behaviour of beryllium is mainly died to its very small size and partly due to its high electronegativity. These two factors increase the polarising power [Ionic charge/ (ionic radii)2] of Be2+ ions to such extent that it becomes significantly equal to the polarising power of Al3+ ions.
Hence the two elements resemble (diagonal relationship) very much.
1. Both of them have the same value of electronegativity (1.5).
2. The standard oxidation potential of Be and Al are of the same order (Be = 1.69 V, Al = 1.7 V)
3. In nature both occur together in beryl, 3BeO, Al2O3, 6SiO2.
4. Due to its small size, beryllium has a high charge density and therefore, exhibits a strong tendency to form covalent compounds. Aluminium too has a strong tendency to form covalent compounds. Thus salts of both beryllium and aluminium have low m.p. are soluble in organic solvents and get hydrolysed by water.
Beryllium does show some tendency to form covalent compounds but other alkaline earth metals do not form covalent compounds.
5. Unlike other alkaline earth metals but like aluminium, beryllium is not easily affected by dry air.
6. Both (Be and Al) do not decompose water even on boiling; because of their weak electropositive character. Other alkaline earth » metals decompose even cold water evolving hydrogen.
7. Beryllium, like aluminium, reacts very slowly with dilute – mineral acids liberating hydrogen.
Be + 2HCl → BeCl2 + H2
2Al + 6HCl → 2AlCl3 + 3H2
Other alkaline earth metals react very readily with dilute acids.
8. The chlorides of both beryllium and aluminium have bridged chloride structures in the vapour phase.
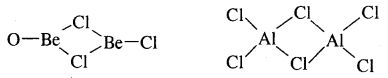
9. Salts of these, metals form hydrated ions e.g., [Be(OH2)4]3+ and [Al(OH2)6]3+ in aqueous solutions.
10. Beryllium and aluminium both react with caustic alkalies to form beryllate and aluminate respectively. Other alkaline earth metals do not react with caustic alkalies.

 Ritan Sheth
Ritan Sheth
