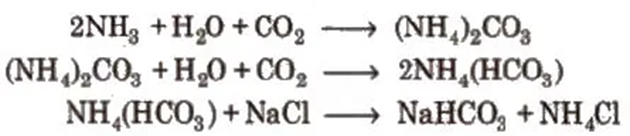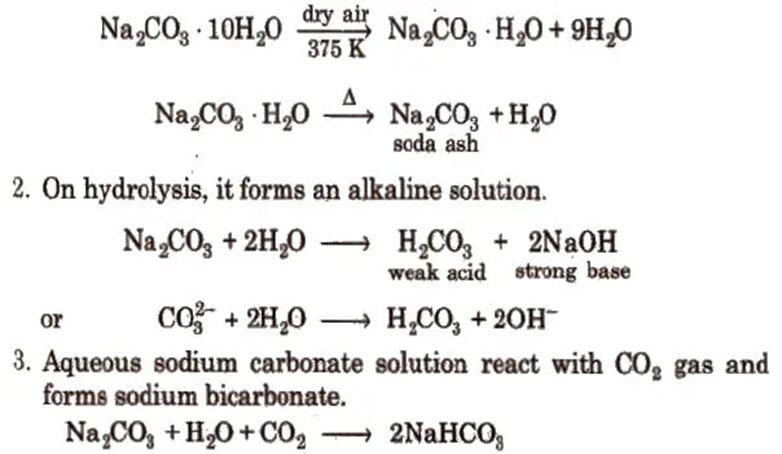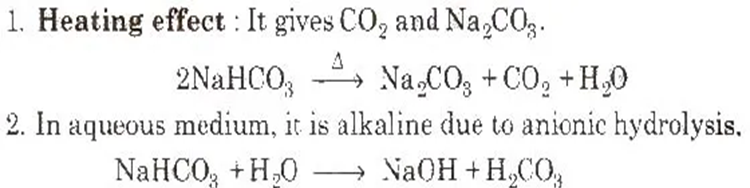1. Group I elements : Alkali metals
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
CHAPTER -10 THE S-BLOCK ELEMENTS
• General Electronic Configuration of s-Block Elements
For alkali metals [noble gas] ns1
For alkaline earth metals [noble gas] ns2
ALKALI METALS
Electronic Configuration, ns1, where n represents the valence shell.
These elements are called alkali metals because they readily dissolve in water to form soluble hydroxides, which are strongly alkaline in nature.
• Atomic and Ionic Radii
Atomic and ionic radii of alkali metals increase on moving down the group i.e., they increase in size going from Li to Cs. Alkali metals form monovalent cations by losing one valence electron. Thus cationic radius is less as compared to the parent atom.
• Ionization Enthalpy
The ionization enthalpies of the alkali metals are generally low and decrease down the group from Li to Cs.
Reason: Since alkali metals possess large atomic sizes as a result of which the valence s-electron (ns1) can be easly removed. These values decrease down the group because of decrease in the magnitude of the force of attraction with the nucleus on account of increased atomic radii and screening effect.
• Hydration Enthalpy
Smaller the size of the ion, more is its tendency to get hydrated hence more is the hydration enthalpy.
Hydration enthalpies of alkali metal ions decrease with increase in ionic sizes.
Li+ > Na+ > K+ > Rb+ > Cs+
• Physical Properties
(i) All the alkali metals are silvery white, soft and light metals.
(ii) They have generally low density which increases down the group.
(iii) They impart colour to an oxidising flame. This is because the heat from the flame excites the outermost orbital electron to a higher energy level. When the excited electron comes back to the ground state, there is emission of radiation in the visible region.
• Chemical Properties of Alkali Metals
(i) Reaction with air:
When exposed to air surface of the alkali metals get tarnished due the formation of oxides and hydroxides.
Alkali metals combine with oxygen upon heating to form different oxides depending upon their nature.
e.g. 4Li + O2 → 2Li2O
Lithium Oxide
2Na + O2 → Na2O2
Sodium Peroxide
K + O2 → KO2
Potassium Superoxide
(ii) Reaction with water:
Alkali metals react with water to form hydroxide and dihydrogen
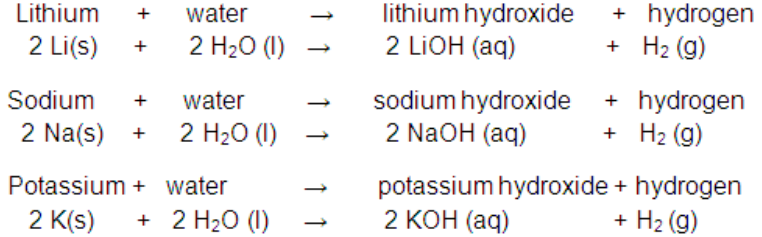
(iii) Reaction with hydrogen:
The alkali metals combine with hydrogen at about 673 K (lithium at 1073 K) to form hydrides.
2M + H2 ————-> 2M+
The ionic character of hydrides increases from Li to Cs.
(iv) Reaction with halogens:
Alkali metals combine with halogens directly to form metal halides.
2M + X2————–> 2MX
They have high melting and boiling points.
Order of reactivity of M:
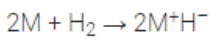
(v) Reducing nature:
The alkali metals are strong reducing agents. In aqueous solution it has been observed that the reducing character of alkali metals follows the sequence Na < K < Rb < Cs < Li, Li is the strongest while sodium is least powerful reducing agent. This can be explained in terms of electrode potentials (E°). Since the electrode potential of Li is the lowest. Thus Li is the strongest reducing agent.
(vi) Solubility in liquid ammonia:
The alkali metals dissolve in liquid ammonia to give deep blue solution. The solution is conducting in nature.
M+ (x + y) NH3 ———-> [M (NH3) X]+ + [e (NH3) y]–
When light falls on the ammoniated electrons, they absorb energy corresponding to red colour and the light which emits from it has blue colour. In concentrated solution colour changes from blue to bronze. The blue solutions are paramagnetic while the concentrated solutions are diamagnetic.
• Uses of Alkali Metals
Uses of Lithium
(i) Lithium is used as deoxidiser in the purification of copper and nickel.
(ii) Lithium is used to make both primary and secondary batteries.
(iii) Lithium hydride is used as source of hydrogen for meteorological purposes.
(iv) Lithium aluminium hydride (LiAlH4) is a good reducing agent.
(v) Lithium carbonate is used in making glass.
Uses of Sodium
(i) Used as sodium amalgum in laboratory (synthesis of organic compounds).
(ii) Sodium is used in sodium vapour lamp.
(iii) In molten state, it is used in nuclear reactors.
(iv) An alloy of sodium-potassium is used in high temperature thermometres.
Uses of Potassium
(i) Salts of potassium are used in fertilizers.
(ii) Used as reducing agent.
Uses of Cesium
(i) In rocket propellent
(ii) In photographic cells.
2. Characteristics of compounds of alkali metals
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
GENERAL CHARACTERISTICS OF THE COMPOUNDS OF ALKALI METALS
(a) Oxides: Alkali metals when burnt in the air form oxides. The nature of oxides depends upon the nature of the alkali metal.
Under ordinary conditions, lithium forms the monoxide (Li2O), sodium forms the peroxide (Na2O2) and the other alkali metals form mainly superoxides (MO2) along with a small number of peroxides.
The increasing stability of the peroxide or superoxide, as the size of the metal ion increases, is due to the stabilization of large anions by larger cations through lattice energy effects. These oxides are easily hydrolysed by water to form the hydroxides according to the following reactions:
M2O + H2O → 2M+ + 2OH–
M2O2 + 2H2O → 2M+ + 2OH– + O2
2MO2 + 2H2O → 2M+ + 2OH– + H2O2 + O2
The oxides and the peroxides are colourless, but the superoxides are yellow or orange coloured. The superoxides are also paramagnetic. Sodium peroxide is widely used as an oxidizing agent in inorganic chemistry.
(b) Hydroxides: Alkali metal hydroxides, MOH are prepared, by dissolving the corresponding oxide in water. Their solubility in the water further increases as we move down the group due to a decrease in lattice energy.
Properties:
1. These are white crystalline solid, highly soluble in water and alcohols. Their solubility in the water further increases as we move down the group due to a decrease in lattice energy.
2. Since alkali metals are highly electropositive, their hydroxides form the strongest bases known. They dissolve in water with the evolution of much heat to give a strongly alkaline solution.
3. They melt without decomposition and are good conductors of electricity in the fused state.
4. These are stable to heat and do not lose water even at red heat. The thermal stability increases on moving from Li to Cs. However, they sublime at about 400°C and the vapours mainly consists of dimers. (MOH)2.
(c) Halides: Alkali metal halides arc prepared by the direct combination of the element, M and halogens. They are normally represented by the formula MX and Cs and Rb, being of large size, also form Polyhalides, i.e. Csl3
Properties:
1. All alkali halides except lithium fluoride are freely soluble in water (LiF is soluble in non-polar solvents).
2. They have high melting and boiling points.
3. Solubility of halides of alkaline metals: The solubility of alkali metal halides show a gradation. For example

4. They are good conductors of electricity infused state.
5. They have an ionic crystal structure. However, lithium halides have a partly covalent character due to polarising power of Li+ ions.
(d) Carbonates and bicarbonates: All alkali metals from carbonates of the type M2CO3. Due to the high electropositive nature of the alkali metals, their carbonates (and also the bicarbonates) are highly stable to heat (however, lithium carbonate decomposes easily by heat. Further, as the electropositive character increases in moving down the group, the stability of carbonates (and bicarbonates) increases in the same order.
Both carbonates and bicarbonates are quite soluble in water and their solubility increases as we move down the group from Li to Cs. Since carbonates are salts of a weak acid (carbonic acid H2CO3), they are hydrolysed in water to give a basic solution.
2M+ + CO3– + H – OH = 2M+ + HCO32- + OH–
Since the alkali metals are highly electropositive, these are the only elements that form stable solid carbonates. However, lithium due to its less electropositive nature does not form solid bicarbonate.
(e) Hydrides: Alkaline metals form hydrides of the type M+N–. The presence of hydrogen as an anion in alkali metal hydrides is evidenced by the fact that on electrolysis hydrogen is liberated at the anode. The hydrides are not very stable. They react with water liberating hydrogen
LiH + H2O → LiOH + H2
These hydrides are, therefore, used as reducing agents. Lithium aluminium hydride, LiAlH4 is even a stronger reducing agent and is used in organic chemistry.
3. Anomalous properties of lithium
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ANOMALOUS BEHAVIOUR OF LITHIUM
Lithium shows anomalous behaviour due to the following reasons:
1. It has the smallest size in its group.
2. It has very high ionization enthalpy and highest electronegativity in the group.
3. Absence of d-orbitals inits valence shell.
As a result, it differs from the other alkali metals in the following properties :
- Lithium is harder than other alkali metals, due to strong metallic bond.
- Lithium combines with O2 to form lithium monoxide, Li2O whereas other alkali metals form peroxides (M2O2) and superoxides (MO2).
- Lithium, unlike the other alkali metals, reacts with nitrogen to form the nitride.
6Li + N2 → 2Li3N
Lithium nitride
- Li2CO3 ,LiF and lithium phosphate are insoluble in water while the corresponding salts of other alkali metals are soluble in water.
- Li2CO3 decomposes on heating to evolve CO2 whereas other alkali metal carbonates do not.
- Lithium nitrate on heating evolves O2 and NO2 and forms Li2O while other alkali metal nitrates on heating form their respective nitrites.
Diagonal Relationship
Lithium shows diagonal resemblance with magnesium [the element of group 2] and this resemblance is due to similar polarising power, i.e.,
[ionic charge / (ionic radius)2] of both these elements.
Lithium resembles magnesium in the following respects :
- The atomic radius of lithium is 1.31 Å while that of magnesium is 1.34 Å.
- The ionic radius of Li+i on is 0.60 Å, which is very close to that of Mg2+ ion (0.65 Å).
- Lithium (1.0) and magnesium (1.2) have almost similar electronegativities.
- Both Li and Mg are hard metals.
- LiF is partially soluble in water like MgF2.
- Both combine with O2 to form monoxides, e.g., Li2O and MgO.
- Both LiOH and Mg(OH)2 are weak bases.
- Both LiCI and MgCl2 are predominantly covalent.
- Both Li and Mg combine with N2 to form their respective nitrides, Li3N and Mg3N2.
Both lithium and magnesium nitrates on heating evolve NO2 and O2 leaving behind their oxides.
4. Some important compounds of sodium
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
SOME IMPORTANT COMPOUNDS OF SODIUM
1. Sodium Chloride, Common Salt or Table Salt [NaCI]
Sea water contains 2.7 to 2.9%by mass of the salt. Sodium chloride is obtained by evaporation of sea water but due to the presence of impurities like CaCl2 and MgCl2 it has deliquescens nature. It is purified by passing HCI gas through the impure saturated solution of
NaCl and due to common ion effect, pure NaCl gets precipitated. 28% NaCl solution is called brine.
2. Sodium Hydroxide or Caustic Soda [NaOH]
Methods of preparation
(i) A 10% solution of Na2CO3 is treated with milk of lime (Causticizing process).
Na2CO3 + Ca(OH)2 → CaCO3 ↓ + 2NaOH
(ii) Electrolytic process involves Nelson cell and Castner-Kellner cell.
A brine solution is electrolysed using a mercury cathode and a carbon anode. Sodium metal discharged at the cathode combines with Hg to form Na-amalgam. Chlorine gas is evolved at the anode.
The amalgam is treated with water to give sodium hydroxide and hydrogen gas.
2Na-Hg + 2H2O → 2NaOH + 2Hg + H2
Physical properties
Sodium hydroxide is a white translucent solid. It is readily soluble in water. Crystals of NaOH are deliquescent.
Chemical properties
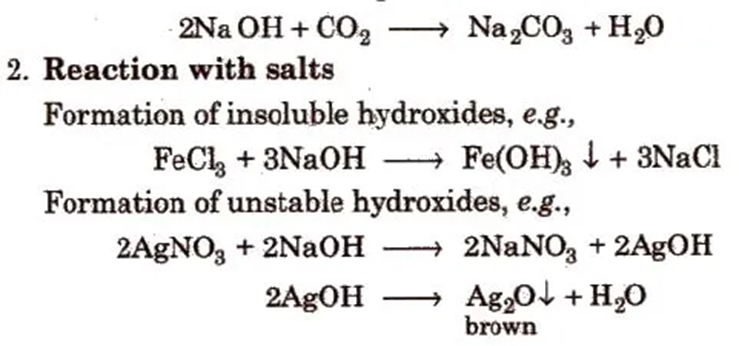
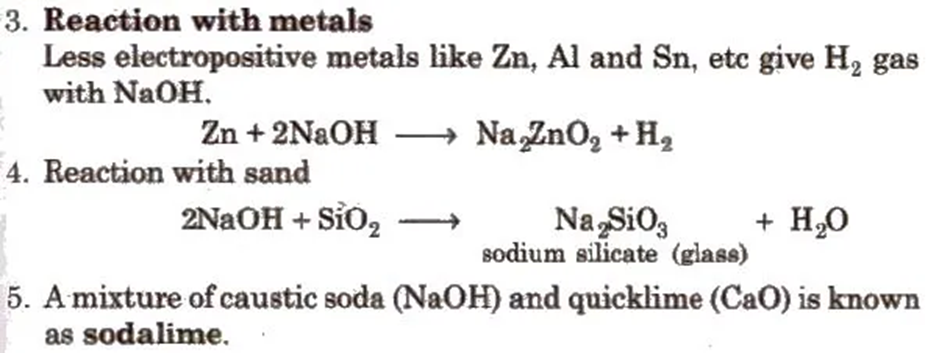
1. It is a hygroscopic, deliquescent white solid, absorbs CO2 and moisture from the atmosphere
3. Sodium Carbonate or Washing Soda (Na2CO3 . 10H2O)
Solvay process
CO2 gas is passed through a brine solution saturated with NH3
Sodium bicarbonate is filtered and dried. It is ignited to give sodium carbonate.
![]()
Properties
1. Sodium carbonate crystallises from water as decahydrate which effloresces on exposure to dry air forming monohydrate which on heating change to anhydrous salt (soda-ash).
Uses
1. It is used in water softening, laundering and cleaning.
2. It is used in paper, paints and textile industries
4. Sodium Bicarbonate or Baking Soda (NaHCO3) Preparation
It is obtained as an intermediate product in Solvay process.
Properties
Uses
1. It is used as a constituent of baking powder which is a mixture of sodium bicarbonate, starch and potassium bitartrate or cream of tartar and in medicine to remove acidity of the stomach (as antacid).
2. NaHCO3 is a mild antiseptic for skin infections.
3 It is used in fire extinguisher.
5. Microcosmic salts [Na(NH4)HPO4 . 4H2O]
Preparation
It is prepared by dissolving Na2HPO4 and NH4Cl in the molecular proportions in hot water followed by crystallisation.
Properties
On heating it forms a transparent glassy bead of metaphosphate, which gives coloured beads of orthophosphates when heated with coloured salts like that of transition metal ions(Cu2+, Fe2+, Mn2+, Ni2+, CO2+).
This test is called microcosmic bead test.
Na(NH4) HPO4 → NH3 + H2O + NaPO3
sodium metaphosphate
CUSO4 → CuO + SO3
CuO + NaPO3 → CuNaPO4 (blue bead)
It is especially used to detect silica which being insoluble in NaPO3 gives a cloudy bead.
5. Biological importance of sodium and potassium
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
BIOLOGICAL IMPORTANCE OF SODIUM AND POTASSIUM
1. Sodium ions help to regulate flow of water across the cell membranes, transportation of sugars and amino acids inside the cells.
2. Potassium ions activate many enzymes, takes part in oxidation of glucose.
3. Potassium ions together with sodium ions are responsible for transmission of nerve signals and maintenance of ionic gradient across the cell.
6. Group II elements : Alkaline earth metals
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ALKALINE EARTH METALS
Alkaline Earth Metals: They were named alkaline earth metals since they were alkaline in nature like alkali metals oxides and they were found in the earth’s crust.
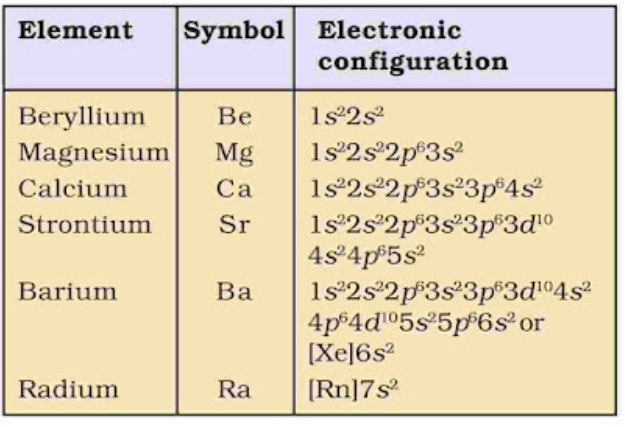
Example, Be (Beryllium), Ca, Mg, Sr etc.
• Electronic Configuration
Their general electronic configuration is represented as [noble gas] ns2.
• Atomic and Ionic Radii
Atomic and ionic radii of alkaline earth metals one comparatively smaller than alkali metals. Within the group atomic and ionic radii increases with the increase in atomic number. Reason: Because these elements have only two valence electrons and the magnitude of the force of attraction with the nucleus is quite small.
• Ionization Enthalpies
These metals also have low ionization enthalpies due to fairly large size of atoms. As the atomic sizes increase down the group ionization enthalpies are expected to decrease in the same manner.
Due to their small size in comparison to alkali metals first ionization enthalpies of alkaline earth metals is higher than that of alkali metals.
• Hydration Enthalpies
The hydration enthalpies of alkaline earth metal ions are larger than those of the alkali metals. Thus alkaline earth metals have more tendency to become hydrate. The hydration enthalpies decreases down the group since the cationic size increases.
Be2+ > Mg2+ > Ca2+ > Sr2+ > Ba2+
Metallic character: They have strong metallic bonds as compared to the alkali metals in the same period. This is due to the smaller kernel size of alkaline earth metal and two valence electrons present in the outermost shell.
• Physical Properties
(i) They are harder than alkali metals.
(ii) M.P and B.P are higher than the corresponding alkali metals due to their small size.
(iii) The electropositive character increases down the group.
(iv) Except Be and Mg, all these metals impart characteristic colour to the flame.
(v) The alkaline earth metals possess high thermal and electrical conductivity.
• Chemical Properties
1. Reaction with oxygen. Beryllium and magnesium are kinetically inert to oxygen because of the formation of a thin film of oxide on their surface.
Reactivity towards oxygen increases as going down the group.
2. Reaction with water. Since these metals are less electropositive than alkali metals, they are less reactive towards water.
Magnesium reacts with boiling water or steam. Rest of the members reacts even with cold water.
Mg + 2H20 ——-> Mg(OH)2 + H2
Ca + 2H20 ————> Ca(OH)2 + H2
3. Reaction with halogens. They combine with the halogens at appropriate temperature to form corresponding halides MX2.
M + X2 ——–> MX2 (X = F, Cl, Br, I)
Thermal decomposition of (NH4)2 BeF4is used for the preparation of BeF2.
4. Reaction with hydrogen. These metals except Be combine with hydrogen directly upon heating to form metal hydrides.
BeH2, however, can be prepared by the reaction of BeCl2 with LiAlH4.
2BeCl2 + LiAlH4 → 2BeH2 + LiCl + AlCl3
7. General characteristics of the compounds of the alkaline earth metals
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
GENERAL CHARACTERISTICS OF COMPOUNDS OF ALKALINE EARTH METALS
Oxides and Hydroxides
(i) The alkaline earth metals bum in oxygen to form MO (monoxide).
(ii) These oxides are very stable to heat.
(iii) BeO is amphoteric in nature while oxides of other elements are ionic.
(iv) Exept BeO, they are basic in nature and react with water to form sparingly soluble hydroxides.
MO + H2O ———> M(OH)2
(v) Hydroxides of alkaline earth metals are less stable and less basic than alkali metal hydroxides.
(vi) Beryllium hydroxide is amphoteric in nature.
Halides
The alkaline earth metals combine directly with halogens at appropriate temperatures forming halides, MX2.
They can also be prepared by the action of halogen acids (HX) on metals, metal oxides, metal hydroxides.
M + 2HX ——-> MX2 + H2
MO + 2HX ——> MX2 + H20
M (OH)2 + 2HX —–> MX2 + 2H20
(i) Except beryllium halides, all other halides of alkaline earth metals are ionic in nature.
(ii) Except BeCl2 and MgCl2 other chloride of alkaline earth metals impart characteristic colours to flame.
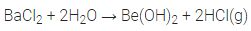
(iii) The tendency to form halide hydrates decreases down the group.
For example, (MgCl2– 8 H20, CaCl2– 6 H20, SrCl2– 6 H20, BaCl2– 2 H2O)
(iv) BeCl2 has a chain structure in the solid phase as shown below.
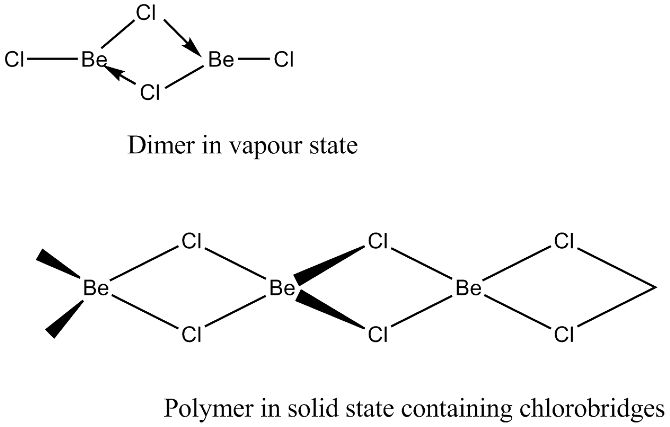
In vapour phase the compound exist as a dimer which decomposes at about 1000K to give monomer in which Be atom is in sp hybridisation state.
Sulphates
(i) The sulphates of alkaline earth metals are white solids and quite stable to heat.
(ii) BeS04 and MgS04 are readily soluble in water. Solubility decreases from BeS04 to BaS04.
Reason. Due to greater hydration enthalpies of Be2+ ions and Mg2+ ions they overcome the lattice enthalpy factor. Their sulphates are soluble in water.
8. Anomalous properties of beryllium
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ANOMALOUS BEHAVIOUR OF BERYLLIUM
The anomalous behaviour of beryllium is mainly died to its very small size and partly due to its high electronegativity. These two factors increase the polarising power [Ionic charge/ (ionic radii)2] of Be2+ ions to such extent that it becomes significantly equal to the polarising power of Al3+ ions.
Hence the two elements resemble (diagonal relationship) very much.
1. Both of them have the same value of electronegativity (1.5).
2. The standard oxidation potential of Be and Al are of the same order (Be = 1.69 V, Al = 1.7 V)
3. In nature both occur together in beryl, 3BeO, Al2O3, 6SiO2.
4. Due to its small size, beryllium has a high charge density and therefore, exhibits a strong tendency to form covalent compounds. Aluminium too has a strong tendency to form covalent compounds. Thus salts of both beryllium and aluminium have low m.p. are soluble in organic solvents and get hydrolysed by water.
Beryllium does show some tendency to form covalent compounds but other alkaline earth metals do not form covalent compounds.
5. Unlike other alkaline earth metals but like aluminium, beryllium is not easily affected by dry air.
6. Both (Be and Al) do not decompose water even on boiling; because of their weak electropositive character. Other alkaline earth » metals decompose even cold water evolving hydrogen.
7. Beryllium, like aluminium, reacts very slowly with dilute – mineral acids liberating hydrogen.
Be + 2HCl → BeCl2 + H2
2Al + 6HCl → 2AlCl3 + 3H2
Other alkaline earth metals react very readily with dilute acids.
8. The chlorides of both beryllium and aluminium have bridged chloride structures in the vapour phase.
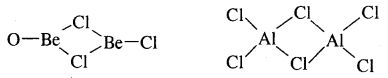
9. Salts of these, metals form hydrated ions e.g., [Be(OH2)4]3+ and [Al(OH2)6]3+ in aqueous solutions.
10. Beryllium and aluminium both react with caustic alkalies to form beryllate and aluminate respectively. Other alkaline earth metals do not react with caustic alkalies.
9. Some important compounds of calcium
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
SOME IMPORTANT COMPOUNDS OF CALCIUM
(i) Calcium Oxide or Quick Lime, CaO

Uses:
(i) In the manufacture of cement, sodium carbonate, calcium carbide etc.
(ii) Used in the purification of sugar.
(iii) In the manufacture of dye stuffs.
(ii) Calcium Hydroxide (Slaked lime), Ca(OH)2
Carbonates
Carbonates of alkaline earth metals are thermally unstable and decompose on heating.
Calcium hydroxide is prepared by adding water to quick lime, CaO. It is a white amorphous powder. It is sparingly soluble in water. The aqueous solution is known as lime water and a
suspension of slaked lime in water is known as milk of lime. When carbon dioxide is passed through lime water it turns milky due to the formation of calcium carbonate.
Uses:
(i) It is used in the manufacturing of building material.
(ii) Used in white-wash as a disinfectant.
(iii) Used to detect C02 gas in the laboratory.
(iii) Calcium Carbonate or Limestone (CaC03)
Preparation: Calcium carbonate occurs in nature in different forms like limestone, marble, chalk etc. It can be prepared by passing C02 through slaked lime in limited amount.
Ca(OH)2 + C02 ——> CaC03 + H20
It can also prepared by the reaction of a solution of sodium carbonate with calcium chloride.
CaCl2 + Na2C03 ——> CaC03 + 2NaCl
Uses:
(i) In the manufacturing of Quick Lime.
(ii) With MgC03 used as flux in the extraction of metals.
(iii) Used as an antacid.
(iv) In the manufacture of high quality paper.
(iv) Calcium Sulphate (Plaster of Paris) CaS04-1/2H20
Preparation: It is obtained when gypsum CaS04– 2 H20 is heated to 393 K
2(CaS04-2H20) ———-> 2(CaS04) . H20 + 3H20
Above 393 K anhydrous CaS04 is formed, which is called ‘dead burnt plaster’.
Properties:
(i) It is a white atmosphous powder.
(ii) When it is mixed in adequate quantity of water it forms a plastic hard mass within 15 minutes.
Uses:
(i) Commonly used in making pottery, ceramics etc.
(ii) Used in the surgical bandages for setting the fractured bone or sprain.
(iii) For making statues, ornamental work, decorative material etc.
(v) Cement
Preparation: Prepared by combining a material rich in CaO with other material such as clay, which contains Si02 along with the oxides of aluminium, iron and magnesium.
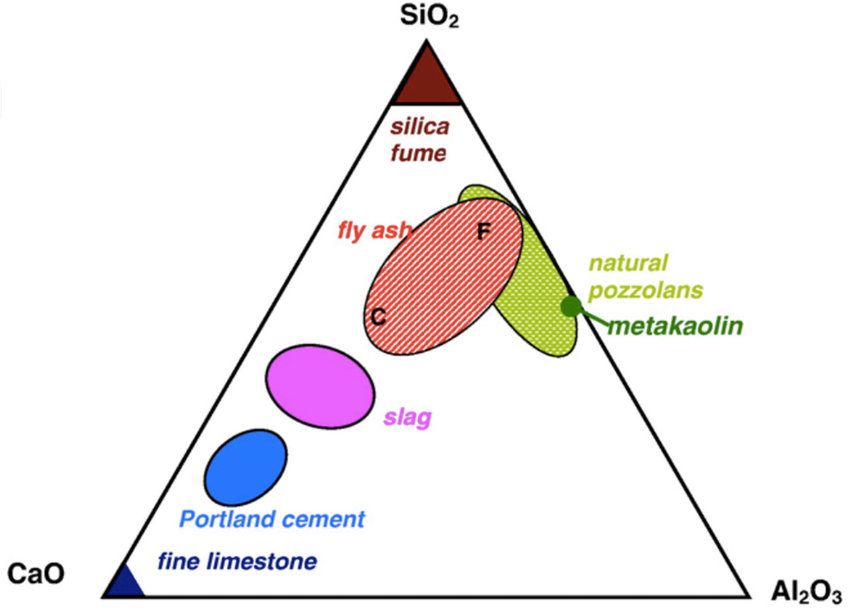
Important Ingredients of portland cement:
(Ca2Si04) dicalcium silicate 26%
(Ca2SiO4) Tricalcium silicate 51%
(Ca3Al206) Tricalcium Aluminate 11%
Uses:
In plastering and in construction purposes.
• s-block elements constitute Group I and II elements.
• General electronic configuration of
Group I = [Noble gas] ns1
Group II = [Noble gas] ns2
• Diagonal Relationship
The first three elements of second period (Li, Be, B) show diagonal similarity with the elements (Mg, Al, Si) of third period. Such similarities are termed as diagonal relationship.
• The alkali metals are silvery-white soft metals. They are highly reactive. Their aqueous solutions are strongly alkaline in nature. Their atomic and ionic sizes increase on moving down the group and ionization enthalpies decrease systematically down the group.
• Alkaline earth metals. They are much similar to alkali metals but due to small size some differences are there. Their oxides and hydroxides are less basic than the alkali metals.
• Sodium hydroxide (NaOH) is prepared by the electrolysis of aq NaCl in Castner- Kellner cell.
Slaked lime Ca(OH)2 is formed by the action of quick lime on water.
• Gypsum is CaS04. 2 H20. On heating upto 390 K CaS04/2H20 (plaster of paris) is formed.
10. Biological importance of magnesium and calcium
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
BIOLOGICAL IMPORTANCE OF MAGNESIUM & CALCIUM
1. An adult body contains about 25g of Magnesium and 1200 g of calcium as compared with only 5g of iron and 0.06g of copper. The daily requirement in the human body has been estimated to be 200-300 mg.
2. All enzymes that utilize ATP in phosphate transfer require magnesium as the cofactor.
3. The main pigment for the absorption of light in plants for photosynthesis is green coloured chlorophyll which contains magnesium.
4. About 99% of body calcium is present in bones and teeth. It also plays important roles in neuromuscular function, interneuronal transmission, cell membrane integrity and blood coagulation. The calcium concentration in plasma is regulated at about 100 mg L-1. It is maintained by two hormones.
5. Calcitonin and parathyroid hormone. Bone is not an inert and unchanging substance but is continuously being solubilized and redeposited to the extent of 400 mg per day in man. All the calcium passes through the plasma.

 Ritan Sheth
Ritan Sheth