- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
WATER
Human body has about 65% water and some plants have as much as 95% water. It is a crucial compound for the survival of all life forms.
Physical Properties of Water
- It is a colourless and tasteless liquid.
- The presence of extensive hydrogen bonding between water molecules leads to high freezing point, high boiling point, high heat of vaporisation and high heat of fusion in comparison to H2S and H2Se.
- Water has a higher specific heat, thermal conductivity, surface tension, dipole moment and dielectric constant, etc.
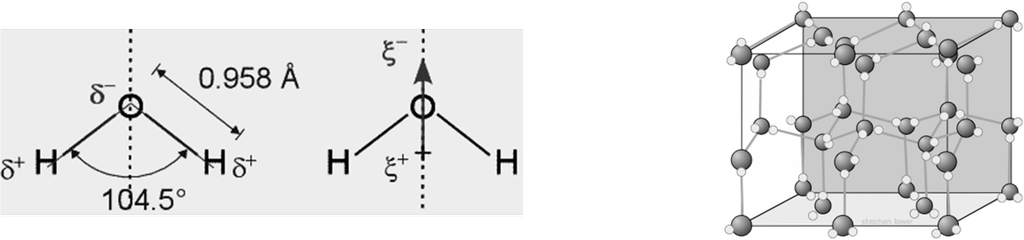
STRUCTURE OF WATER
CHEMICAL PROPERTIES OF WATER
(1) Amphoteric Nature:
H2O(l) + NH3(aq) ⇌ OH-(aq) + NH4+(aq)
H2O(l) + H2S(aq) ⇌ H3O+(aq) + HS-(aq)
The auto-protolysis (self-ionization) of water takes place as follows:
H2O(l) + H2O(l) ⇌ H3O+(aq) + OH-(aq)
(2) Redox Reactions: Water is easily reduced to hydrogen by highly electropositive metals.
2H2O(l) + 2Na(s) → 2NaOH(aq) + H2(g)
With fluorine also it is oxidised to O2.
2F2(g) + 2H2O(l) ® 4H+ (aq) + 4F–(aq) + O2(g)
(3) Hydrolysis Reaction: Due to high dielectric constant, it has a very strong hydrating tendency..
P4O10(s) + 6H2O(l) → 4H3PO4(aq)
SiCl4(l) + 2H2O(l) → SiO2(s) + 4HCl(aq)
(4) Hydrates Formation:
(i) coordinated water e.g. [Cr(H2O)6]Cl3
(ii) interstitial water e.g., BaCl2.2H2O
(iii) hydrogen-bonded water eg. [Cu(H2O)4]SO4.H2O
HARD AND SOFT WATER
- Temporary hardness,
- Permanent hardness.
Temporary Hardness due to the presence of magnesium and calcium bicarbonates. It can be removed by:
(i) Boiling:
Mg(HCO3)2 ⎯ heating® Mg(OH)2 ↓ + 2CO2 ↑
Ca(HCO3)2 ⎯ heating® CaCO3 ↓ +H2O+ CO2 ↑
(ii) Clark’s method:
Ca(HCO3)2 + Ca(OH)2 ® 2CaCO3 ↓ +2H2O
Mg(HCO3)2 +2Ca(OH)2 ®2CaCO3¯ +Mg(OH)2¯ +2H2O
Permanent Hardness is due to the presence of soluble salts of magnesium and calcium in the form of chlorides and sulphates in water. Permanent hardness cannot be removed by boiling. It can be removed by the following methods.
(i) Treatment with washing soda (sodium carbonate):
MCl2 + Na2CO3 ® MCO3↓ + 2NaCl (M=Mg, Ca)
MSO4 + Na2CO3 ® MCO3↓ + Na2SO4
(ii) Calgon’s method: Sodium hexametaphosphate (Na6P6O18), commercially called ‘calgon’, when added to hard water, the following reactions take place.
Na6P6O18 ® 2Na+ +Na4P6O18-2 (M = Mg, Ca)
M+2 +Na4P6O18-2 ® Na2MP6O18-2 +2Na+ the complex anion keeps the Mg2+ and Ca2+ ions in solution
(iii) Ion-exchange method: sodium aluminium silicate (NaAlSiO4) can be written as NaZ. When this is added in hard water, exchange reactions take place.
2NaZ(s) +M+2(aq) ® MZ2(s) +2Na+(aq) (M=Mg, Ca)
Permutit/zeolite is said to be exhausted when all the sodium in it is used up. It is regenerated for further use by treating with an aqueous sodium chloride solution.
MZ2(s) +2NaCl(aq) →2NaZ(s) + MCl2
Hydrogen Peroxide (H2O2)
PREPARATION
(i) Acidifying barium peroxide and removing excess water by evaporation under reduced pressure gives hydrogen peroxide.
BaO2.8H2O(s) + H2SO4(aq) ® BaSO4(s) + H2O2(aq) + 8H2O(l)
(ii) Peroxodisulphate, obtained by electrolytic oxidation of acidified sulphate solutions at high current density, on hydrolysis yields hydrogen peroxide.
2HSO4-(aq) - electrolysis ® HO3SOOSO3H(aq) -hydrolysis ® 2HSO4-(aq) + 2H+(aq) + H2O2(aq)
(iii) Industrially it is prepared by the auto-oxidation of 2-alklylanthraquinols.
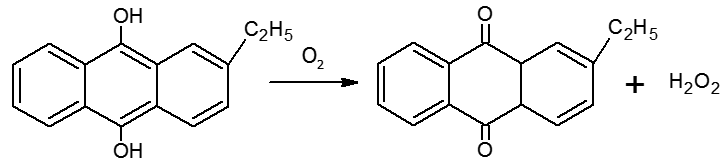
In this case 1% H2O2 is formed. It is extracted with water and concentrated to ~30% (by mass) by distillation under reduced pressure. It can be further concentrated to ~85% by careful distillation under low pressure. The remaining water can be frozen out to obtain pure H2O2
PHYSICAL PROPERTIES
In the pure state H2O2 is an almost colourless (very pale blue) liquid. H2O2 is miscible with water in all proportions and forms a hydrate H2O2.H2O (M.P. 221K). A 30% solution of H2O2 is marketed as ‘100 volume’ hydrogen peroxide. It means that one millilitre of 30% H2O2 solution will give 100 V of oxygen at STP. Commercially, it is marketed as 10 V, which means it contains 3% H2O2.
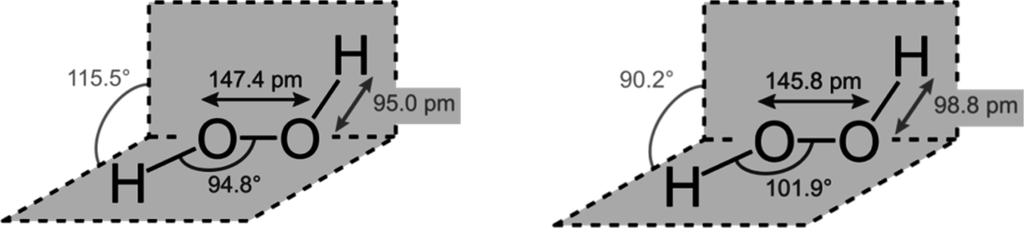
STRUCTURE
Hydrogen peroxide has a non-planar structure. The molecular dimensions in the gas phase and solid phase are shown:
CHEMICAL PROPERTIES
It acts as an oxidising as well as reducing agent in both acidic and alkaline media. Simple reactions are described below.
(i) Oxidising action in acidic medium
2Fe+2(aq) + 2H+(aq) +H2O2(aq) ® Fe+3(aq) + 2H2O
PbS(s) + 4H2O2(aq) ® PbSO4(s) + 4H2O
(ii) Reducing action in acidic medium
2MnO4-(aq) + 5H2O2(l) + 6H+ ® 2Mn2+(aq) + 5O2(g) + 8H2O
HOCl + H2O2 ®H3O+ + Cl- + O2
(iii) Oxidising action in basic medium
2Fe+2 + H2O2 ® Fe+3 + 2OH-
Mn+2 + H2O2 ® Mn+4 + 2OH-
(iv) Reducing action in basic medium
I2 + H2O2 + 2OH- ® 2I- + 2H2O + O2
2MnO4- + 3H2O2 ® 2MnO2 +3O2 + 2H2O +2OH-
STORAGE
H2O2 decomposes slowly on exposure to light.
2H2O2(l) ® 2H2O(l) + O2(g)
In the presence of metal surfaces or traces of alkali (present in glass containers), the above reaction is catalysed. It is, therefore, stored in wax-lined glass or plastic vessels in dark. Urea can be added as a stabiliser. It is kept away from dust because dust can induce explosive decomposition of the compound.
Uses
Its wide scale use has led to tremendous increase in the industrial production of H2O2. Some of the uses are listed below:
(i) In daily life it is used as hair bleach and as a mild disinfectant. As an antiseptic it is sold as perhydrol.
(ii) It is used to manufacture chemicals like sodium perborate and per-carbonate, which are used in high quality detergents.
(iii) It is used in the synthesis of hydroquinone, tartaric acid and certain food products and pharmaceuticals (cephalosporin) etc.
(iv) It is employed in the industries as a bleaching agent for textiles, paper pulp, leather, oils, fats, etc.
(v) Nowadays it is also used in Environmental (Green) Chemistry. For example, in pollution control treatment of domestic and industrial effluents, oxidation of cyanides, restoration of aerobic conditions to sewage wastes, etc.
Heavy Water, D2O
It is extensively used as a moderator in nuclear reactors and in exchange reactions for the study of reaction mechanisms. It can be prepared by exhaustive electrolysis of water or as a by-product in some fertilizer industries.
It is used for the preparation of other deuterium compounds, for example:
CaC2 +D2O ® C2D2 + Ca(OD)2
SO3 + D2O ® D2SO4
Al4C3 + 12D2O ® 3CD4 + 4Al(OD)3

 Kaysons Publication
Kaysons Publication
