- Books Name
- CBSE Class 7 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 7
- Subject
- Science
RUSTING OF IRON
When iron objects are left exposed to moist air (oxygen and water both), a substance with a brown flaky layer is observed on their surfaces. This brown flaky layer is hydrated iron oxide. It is called rust.
Rust falls off the surface, exposing the iron surface beneath. Rusting of iron is a slow change that destroys the whole iron object.
Iron is an important metal. It is used in making bridges, cars, ships, trucks, gates, benches and various other useful articles. Every year, a lot of monetary loss occurs due to damage of iron articles by rusting.
The process of rusting of iron is represented as:
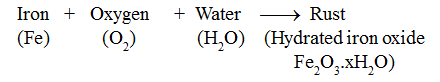
Remember
• Rusting of iron takes place in the presence of both oxygen and water (or water vapour). If anyone of these is not present, rusting will not occur.
Advance Learning
• Rusting of iron becomes faster if the content of moisture in the air increases.
• Rusting is faster in salty water.
Prevention of Rusting of Iron
Rusting of iron can be prevented in many ways.
(i) By avoiding direct contact with air and moisture :
It is done by using the following methods :
• Applying grease or oil on the exposed parts of iron articles.
• Painting the surface of iron articles.
• Galvanizing the surface of iron articles. Galvanization is a process in which a layer of metals like chromium or zinc is deposited on the surface of iron articles electrolytically, i.e., by passing electric current.
• Electroplating the surface of iron articles with metals, which are not attacked by atmospheric moisture. The shining parts of bicycles are given a coating of chromium (chrome plating) to protect them from rusting.
(ii) By alloying : When mixed with certain corrosion resistant metals or some non-metals, iron forms alloys which are resistant to rusting. Stainless steel, an alloy of iron, nickel and chromium does not rust.
Advance Learning
• Electroplating
It is the deposition of a metallic coating (say gold) by passing electric current through a solution containing dissolved metal ions and the metal object to be electroplated.
This is the process by which wrist watches, jewellery and other items are plated with gold.

 Grow Career Publication
Grow Career Publication
 Param Publication
Param Publication
