- Books Name
- CBSE Class 6 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 6
- Subject
- Science
TYPES OF CHANGES
(i) Reversible and irreversible changes
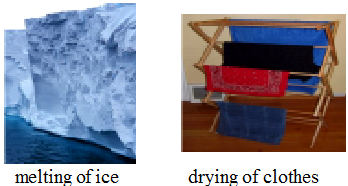
(a) Reversible change
A change which can be reversed by the conditions is called a reversible change.
eg. Melting of ice, dissolving of salt in water, drying of clothes etc.
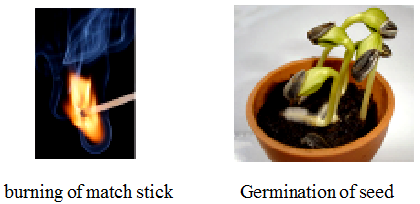
(b) Irreversible change
A change that cannot be reversed by reversing the condition is called an irreversible change.
eg. Burning of paper, curdling of milk
Activity–1
AIM : To find out weather the change can be reversed or not.
Some common changes
Change Can be Reversed
Raw egg to boiled egg Yes/No
Batter to idli
Wet clothes to dry clothes
Woollen yarn to knitted sweater
Grain to its flour
Cold milk to hot milk
Straight string to coil string
Bud to flower
Milk to paneer
Cow dung to biogas
Stretched rubber band to its normal size
Solid ice cream to molten
Some common changes are given in above table. We have to find that the changes given can be reversed or not.
The answer of changes which can be reversed or not is given in Yes/no in the table below.
Some common changes
Change Can be Reversed
Raw egg to boiled egg No
Batter to idli No
Wet clothes to dry clothes Yes
Woollen yarn to knitted sweater Yes
Grain to its flour No
Cold milk to hot milk Yes
Straight string to coil string Yes
Bud to flower No
Milk to paneer No
Cow dung to biogas No
Stretched rubber band to its normal size Yes
Solid ice cream to molten Yes
- Books Name
- Class 6 Science Book
- Publication
- PathSet Publications
- Course
- CBSE Class 6
- Subject
- Science
INTRODUCTION
Everything around us is undergoing a process of change. Our hair and nails keep growing. Leaves die and new leaves take their place. While some changes in our environment are temporary and can change back to their original positions, other changes are relatively permanent.
We can bring about a change in a substance by doing one or more of the following processes:
- Heating.
- Applying force.
- Mixing it with something else.
CHANGE
- Change is anything that becomes different.
- If something new is formed that means a change has occurred.
- For Example: - When we burn a piece of paper it will turn to ashes. This shows a big change has occurred as ashes are very different in colour, size or texture.

Changes caused by heating: When an object is heated, it gets affected in one or many possible ways.
- Some objects get hot but do not change in any other way.
- Some objects get hot and also expand in size.
- Some objects get hot and begin to bum.
- Some objects get hot and change their state.
Changes by applying pressure: When we apply force to an object,
- We can change its shape and size.
- Air can be compressed.
- Metals can be hammered into thin sheets.
- Elastic can be stretched.
- Cotton can be spun into thin threads.
Changes by mixing a substance with other: We can bring about a change in a substance by mixing it with another. For example, making solution by mixing water soluble substances in water.
Metals expand on heating and contract on cooling.
Chemical changes: These are the changes in which chemical properties of a substance change, and a new substance is formed. For example, cooking of food.
Physical changes: These are the changes in which only physical property of a substance changes and no new substance is formed.
Characteristics of physical changes:
- No new substances are formed.
- Products are identical to the reactants.
- These changes are reversible.
Characteristics of chemical changes:
- Properties of products are different from the properties of reactants.
- Most of the chemical changes are irreversible.
- These changes always result in energy changes.
Reversible changes: These are the changes that can be reversed. For example, stretching of rubber.
Irreversible changes: These are the changes which cannot be brought back to its original state. For example, burning of paper.
Natural changes: The changes which occur in nature on their own are called natural changes. For example, change of day and night, change of season.
Slow changes: The changes which take longer time to occur are called slow changes. For example, rusting of iron, tooth decay.
Contraction: A process in which an object becomes smaller or shrinks is called contraction.
Evaporation: A process in which liquid changes into vapour is called evaporation.
Expansion: A process in which an object becomes bigger in size, e.g., metals expand on heating.
Melting: A process in which a solid melts to become a liquid on heating is called melting.
Can all changes always be Reversed?
On this basis, changes around us can be classified into two broad categories:
- Reversible changes
- Irreversible changes
Reversible changes can be described as changes that can be reversed by reversing the action or changing the conditions. Example: freezing of water, rolling of a chapati from dough etc.
Irreversible changes can be described as changes that cannot be reversed even after bringing about changes in the conditions. Example: rusting of iron, cooking of vegetables etc.

Figure 1 Closing and opening of mimosa leaves represent a reversible change
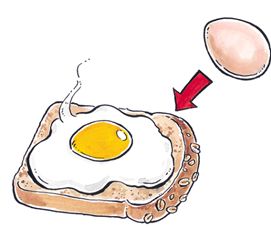
Figure 2 Cooking of an egg into an omelette represents an irreversible change
Substances and materials usually undergo two major types of changes:
- Physical change: This represents a change not in the chemical identity but the physical form of a substance. When substances undergo a physical change, there is no formation of a new substance and more or less these changes can be reversed. Example: boiling of water and melting of ice represent reversible physical changes while growing of height is an irreversible physical change.

Figure 3 Physical Change
- Chemical change: This represents a change in the chemical identity of a substance. These are irreversible changes because the original substance gets converted into a new substance and cannot be brought back. Example: cooking of rice, burning of matchstick etc.

Figure 4 Chemical Change
Difference between physical and chemical changes:
Physical Change
Chemical Change
A change in matter which occurs without causing any change in the composition of the matter is known as physical change
While a chemical change is defined as the change in the chemical composition of matter
Usually, physical changes are reversible in nature
While chemical changes are often irreversible
No new products are formed when an object undergoes physical change
Chemical changes often lead to formation of new products
These changes have no impact on the molecular composition of the substance
Chemical changes have a direct impact on the chemical bonds and molecular composition of a substance

 Param Publication
Param Publication
 PathSet Publications
PathSet Publications
