Matter (Solid, Liquid or Gas)
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Chapter:- 2
Is Matter around us Pure
Matter (Solid, Liquid or Gas)
- Pure Substance,
- Mixtures (No Fixed Composition)
Pure Substance
- Elements (Cannot be broken down to simpler substances)
Examples:-
Copper, oxygen, iron, hydrogen, mercury etc.
- Compounds (Have fixed composition can be broken down into elements by chemical or electrochemical reactions)
Examples:-
Water, methane, sugar, salt etc.
Mixtures (No Fixed Composition)
- HomogeneousUniform composition
- HeterogeneousNon-Uniform composition
Homogeneous (Uniform composition)
Examples:-
Sugar in water, salt in water, sulphur in carbon
disulphide, water in alcohol etc.
Heterogeneous Non-Uniform composition
Examples:-
Sand and salt, sugar, and salt water in oil etc.
Mixtures
When two or more substances (elements/ compounds) are mixed together in any ratio, but they do not combine chemically, a mixture is formed.
Types of Mixtures
Depending upon the nature of the components that form a mixture, we have two types of mixtures.
Mixtures (No Fixed Composition)
- Homogeneous
- Heterogeneous
Homogeneous Mixtures
Air, alloy, soda water etc.

Heterogeneous Mixtures
A mixture of oil and water suspensions colloids etc

Components of a Solution
- Solvent
- Solute
Solvent
The component of the solution that dissolves the other component in it (usually the component present in larger amount) is called the solvent.
Solute
The component of the solution that is dissolved in the solvent (usually present in lesser quantity) is called the solute.
Types of Solution
Depending upon the amount of solute present in a given amount of solvent, the
solution can be classified into following three classes
Types of Solution
- Saturated Solution
- Unsaturated Solution
- Supersaturated solution
Saturated Solution
A solution in which no more amount of solute can be dissolved at a given temperature is known as saturated solution. The amount of solute present in the saturated solution at this temperature is called its solubility.
![]()

Unsaturated Solution
Some more amount of solute can be dissolved at a given temperature, is called unsaturated solution.
Supersaturated Solution If solution contains more amount of solute than the saturation concentration, it is called supersaturated solution.

Properties of a Solution
- A solution is a homogeneous mixture.
- The particles of a solution are smaller than 1 nm (10-9m) in diameter. therefore, they cannot be seen by naked eyes.
- Due to very small particles size, they do not scatter a beam of light passing through the solution. So, the path of light is not visible in a solution.
- A solution is stable. The solute particles cannot be separated by the process of filtration, also they do not settle down when left undisturbed.
Concentration of a Solution
The amount of solute present in a given amount of solution.

Suspension and Evaporation
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Suspension and Evaporation
Suspension
A suspension is a heterogeneous mixture in which the solute particles do not dissolve but remain suspended throughout the bulk of the medium.
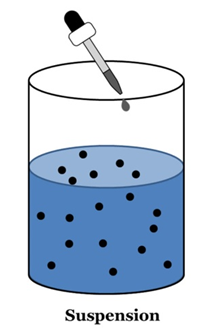
Properties of a Suspension
- Suspension is a heterogeneous mixture.
- Its particles can be seen by naked eyes.
- Its particles scatter a beam of light passing and make its path visible (Tyndall effect).
- It is unstable. The soluble particles settle suspension is left undisturbed or they can by the process of filtration.
Colloidal Solution

A colloid (or colloidal solution) is a mixture that is heterogeneous but appears to be homogeneous as the articles are uniformly spread
throughout the solution. e.g., milk, shaving cream, cheese etc.
Properties of a Colloid
- A colloid appears to be homogeneous but actually it is heterogeneous.
- A colloid appears to be homogeneous but actually it is heterogeneous.
- The size of particles of colloid is very small. They can not be seen even with a microscope.
- Its particles can pass through filter paper, therefore, a colloid cannot be separated by filtration. However, they get separated by a special technique, called centrifugation.
Some Common Examples of Colloids
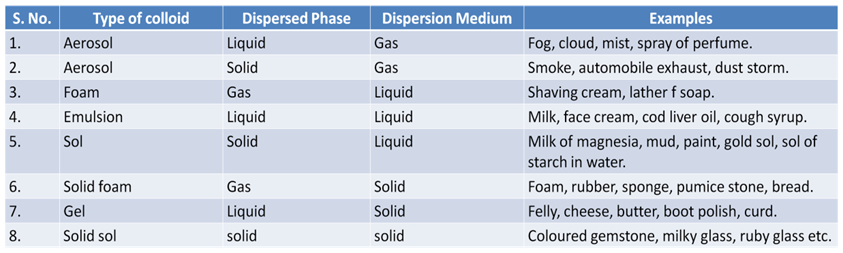
Components of a Colloid
The solute like component form the dispersed phase and the component in which the dispersed phase is suspended is known as the dispersion medium.
Evaporation
This method can be used to separate a volatile component (solvent) from a non-volatile component of a mixture.
Centrifugation
(Separation of Cream from Milk)
Two components having difference in densities can be separated by centrifugation.
Illustration
How the separation of cream from milk takes place?
Solution
To separate cream from milk, milk is churned for 2-3 minutes. Cream collects at the centre and being lighter than milk floats at the top of the mixture.
Decantation
This method is also applied where a difference between densities of components lies. A less soluble solid from a liquid can be separated by first keeping the mixture undisturbed and then decanting the liquid slowly leaving the solid in the first container.
Separating Funnel
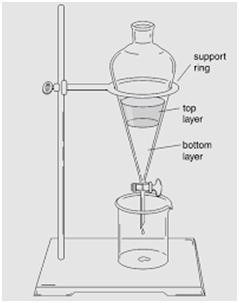
(Separation of Mixture of Two Immiscible Liquids)
This method is used for separating two immiscible liquids and is also based on the difference in densities.
Illustration
Name the apparatus used to separate these two liquids.
Solution
Separating funnel in used to separate oil from water.
Illustration
State the principle involved in this process.
Solution
This process is based upon the principle that immiscible liquids are separated out in layers depending upon their densities.
Illustration
How the layer is formed in separating funnel?
Solution
Kerosene oil being lighter will form the upper layer while water being heavier will form the lower layer.
Illustration
A mixture containing two liquids is placed in separating funnel. Answer the following.
- What type of liquids form the mixture?
- Which of the liquids will form the lower layer?
- What is the basis of this method?
Solution
- Two liquids which are immiscible with each other form the mixture.
- The heavier liquid will form the lower layer.
- The method is based on
(a):- mutual immiscibility of the two liquids
(b):- difference in the densities of the two liquids.
Sublimation and Distillation
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Sublimation and Distillation
Sublimation
(Separation of a Mixture of Salt and Ammonium Chloride)
Some solids have a tendency to sublime on heating, i.e., they convert directly from solid to gaseous/vapour phase on heating through the liquid phase.
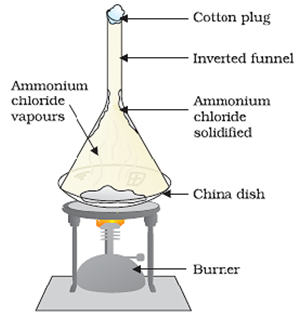
Chromatography
(Separation of Two Colour of a Dye)
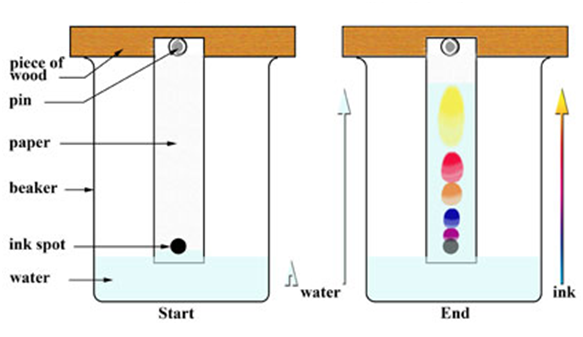
This method is used to separate a mixture of different dyes. The coloured component that is more soluble in water, rises faster and in thisway the colours get separated. This technique is generally used for the separation of those solutes that dissolve in the some solvent.
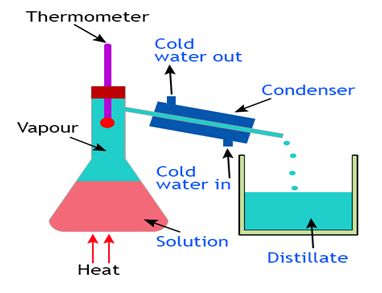
Distillation
(Separation of two Miscible Liquids)
Two miscible liquids that boil without decomposition and have sufficient difference in their boiling point (> 25° C) can la separated by simple distillation. Fractional distillation is used to separate a mixture of miscible liquids the difference it the boiling point of whichis less than 25° C.
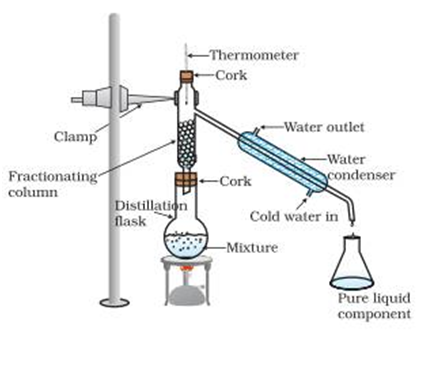
Fractional Distillation
More than components can be separated. Difference in boiling points of components may be less than 25oC. A long fractionating column is also used (where evaporation and condensation take place side by side) along with distillation flask distillation flask, condenser and receiver.
Separation of Different Gases Present in Air
Air is a homogeneous mixture of a number of gases. These can be separated from air by fractional distillation.
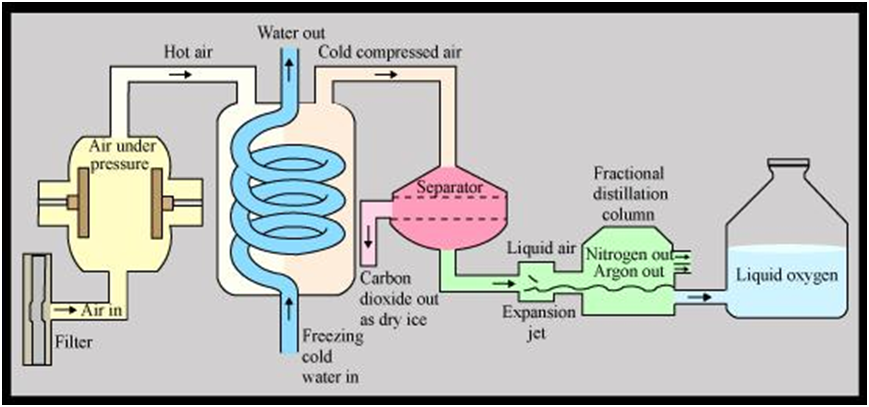
Flow diagram showing the separation of gases of air
Air (Free from CO2 and water vapours) High pressure and low (temperature)
- Liquefied Air (BP = – 200oC)Fractional distillation (Slow warming in fractionating column)
- Nitrogen Separates firs(BP = – 196oC)
- Oxygen (BP = – 183oC) Separation of constituents of air
Crystallisation
(Separation of Pure Substance from its impure Form)
Crystals are the purest form of a substance and have definite geometrical shapes. The process by which an impure compound is converted into its crystals is known as crystallisation.
Advantages of Crystallisation over Evaporation
Crystallisation is a better technique than evaporation to purify a solid due to the following reasons.
- During evaporation, the solution is heated to dryness. Some solids may decompose or some substances like sugar may get charred during direct heating.
- Some impurities may still be present in the solution after filtration and they make the solid impure.
- Direct heating or evaporation does not give crystals. Only a solid residue is left in the dish.
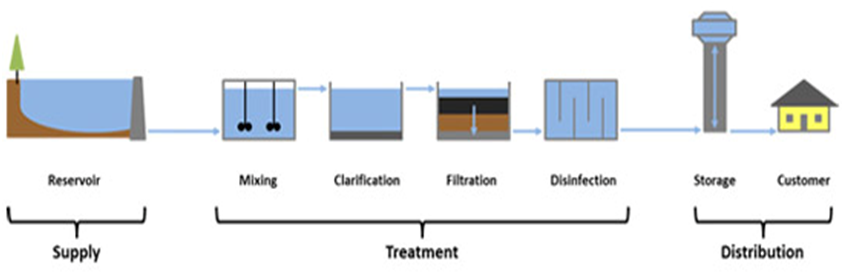
Purification of Drinking Water
In cities, drinking water is supplied from water works. The various processes used in water works for purification are:-
- Sedimentation to remove suspended solids.
- Loading with alum to remove negatively charged colliodal clay particles.
- Filtration to remove dissolved solids.
- Chlorination to kill bacteria.
Purification of Drinking Water
Pure Substance
A substance that consists of only a single type of constituent particles is called pure substance. e.g., gold, water etc.
Element
An element consists of only one type of atoms. e.g., gold, silver, hydrogen, oxygen etc. An element is a pure substance.
Elements are further classified into three groups: metals, non-metals and metalloids.
Metals and Non metals
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Metals and Non metals
Metals (Properties)
- These are lustrous.
- These are silver-gray or golden yellow in colour.
- Metals are malleable and ductile.
- Metals are good conductors of heat and electricity.
- Metals are sonorous, i.e., produce a ringing sound when hit.
Non-Metals (Properties)
- These are non-lustrous.
- The non-metals display a variety of colour.
- Non-metals are neither malleable nor ductile, but they are brittle.
- Non-metals are poor conductors of heat and electricity.
- Non-metals are non-sonorous.
Metalloids
Elements having intermediate properties between those of metals and non-metals are called metalloids. e.g., boron, silicon, germanium etc.
Compounds
A compound is a substance composed of two or more elements, chemically combined with one another in a fixed proportion. e.g., water, methane etc.
Suspension and Evaporation
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Suspension and Evaporation
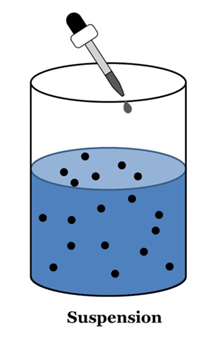
Suspension
A suspension is a heterogeneous mixture in which the solute particles do not dissolve but remain suspended throughout the bulk of the medium.
Properties of a Suspension
- Suspension is a heterogeneous mixture.
- Its particles can be seen by naked eyes.
- Its particles scatter a beam of light passing and make its path visible (Tyndall effect).
- It is unstable. The soluble particles settle suspension is left undisturbed or they can by the process of filtration.
Colloidal Solution

A colloid (or colloidal solution) is a mixture that is heterogeneous but appears to be homogeneous as the articles are uniformly spread
throughout the solution. e.g., milk, shaving cream, cheese etc.
Properties of a Colloid
- A colloid appears to be homogeneous but actually it is heterogeneous.
- A colloid appears to be homogeneous but actually it is heterogeneous.
- The size of particles of colloid is very small. They can not be seen even with a microscope.
- Its particles can pass through filter paper, therefore, a colloid cannot be separated by filtration. However, they get separated by a special technique, called centrifugation.
Some Common Examples of Colloids
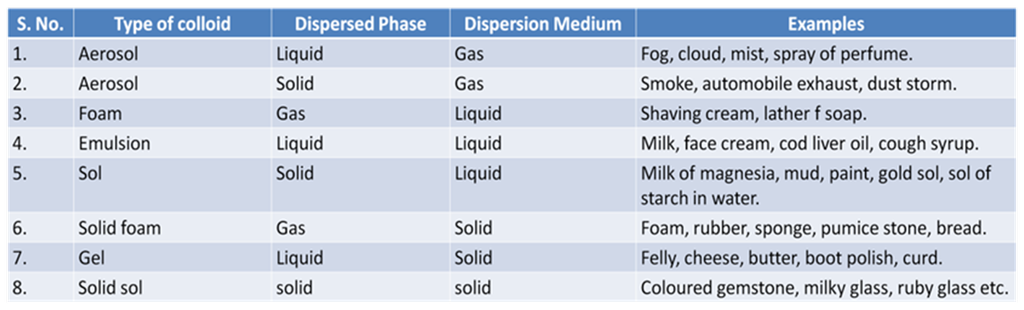
Components of a Colloid
The solute like component form the dispersed phase and the component in which the dispersed phase is suspended is known as the dispersion medium.
Evaporation
This method can be used to separate a volatile component (solvent) from a non-volatile component of a mixture.
Centrifugation
(Separation of Cream from Milk)
Two components having difference in densities can be separated by centrifugation.
Decantation
This method is also applied where a difference between densities of components lies. A less soluble solid from a liquid can be separated by first keeping the mixture undisturbed and then decanting the liquid slowly leaving the solid in the first container.
Separating Funnel
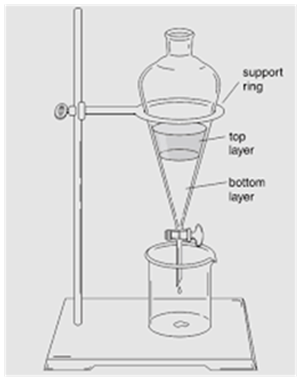
(Separation of Mixture of Two Immiscible Liquids)
This method is used for separating two immiscible liquids and is also based on the difference in densities.

 Kaysons Publication
Kaysons Publication
