- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
VAPOUR PRESSURE
Vapor pressure is the pressure of the vapor over a liquid (and some solids) at equilibrium.
1) Imagine a closed box of several liters in size. It has rigid walls and is totally empty of all substances.
2) Now, I inject some liquid into the box, but the box is not full of liquid. What will happen to the liquid?
3) That's right. Some, maybe all, of the liquid will evaporate into gas, filling the empty space. Now if all the liquid evaporates, we just have a box of gas. That's not what we want. So let's suppose that only some of the liquid evaporated and that there are both the liquid state and the gas state present in the box.
The gas that is above the liquid is called its vapor and it creates a pressure called vapor pressure.
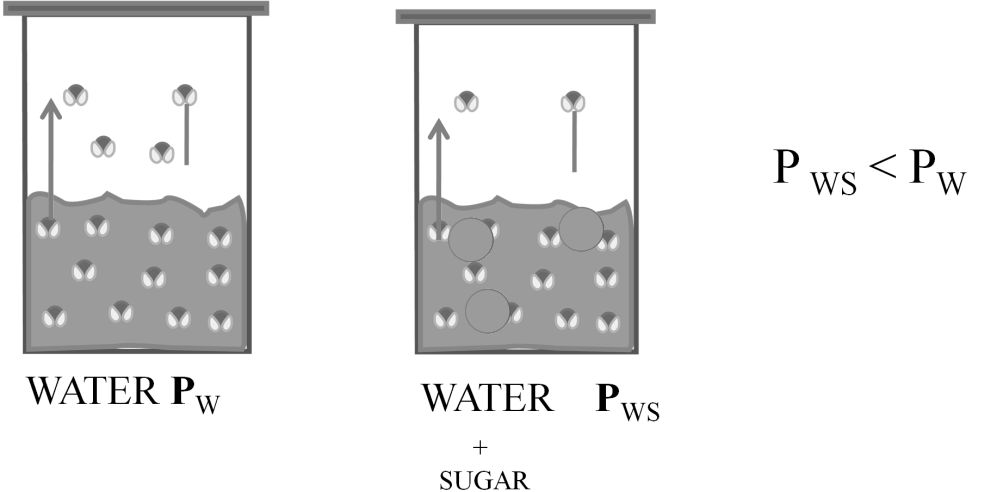
RAOULT’S LAW
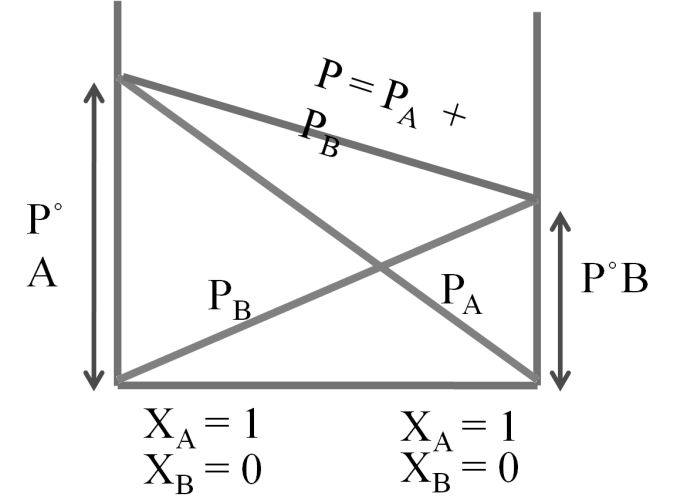
When two liquids (A&B) are mixed.
Partial pressure of A = PPA = XA P°A
Partial pressure of B = PPB = XB P°B
P°A = vapour of pressure of pure A
P°B = vapour Pressure Of pure B
Total pressure P = PPA + PPB ® Dalton’s law
P = P°A XA + P°B XB ® Raoult’s law
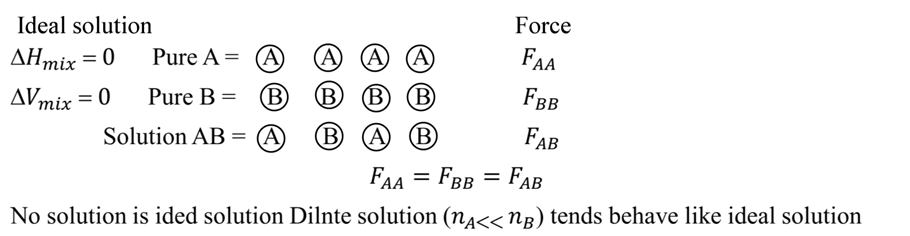
Ideal and non-ideal solutions

Non ideal solutions
For a non-ideal solution
(1) DHmix ¹ 0 (It may be less or more)
(2) DVmix ¹ 0 (It may be less or more)
(3) Does not follow Raoult’s law.
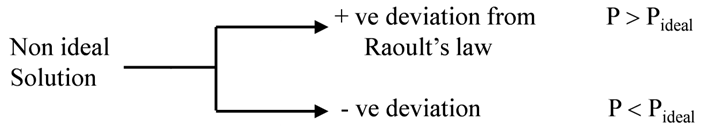
Non ideal solutions
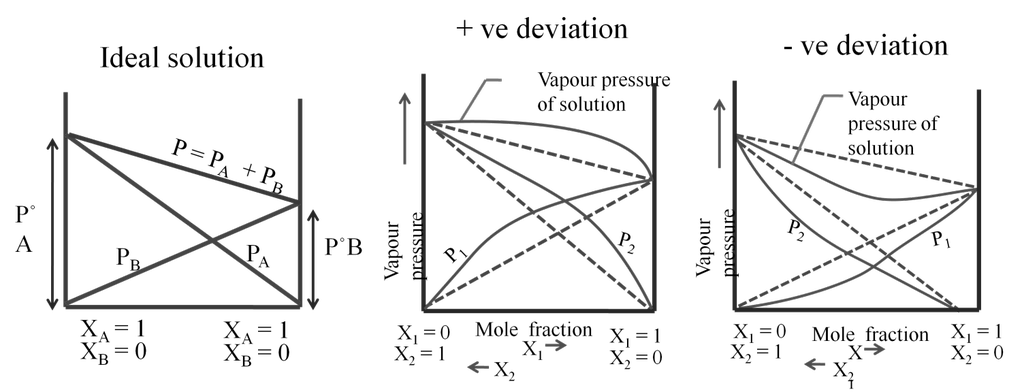
Non ideal solutions

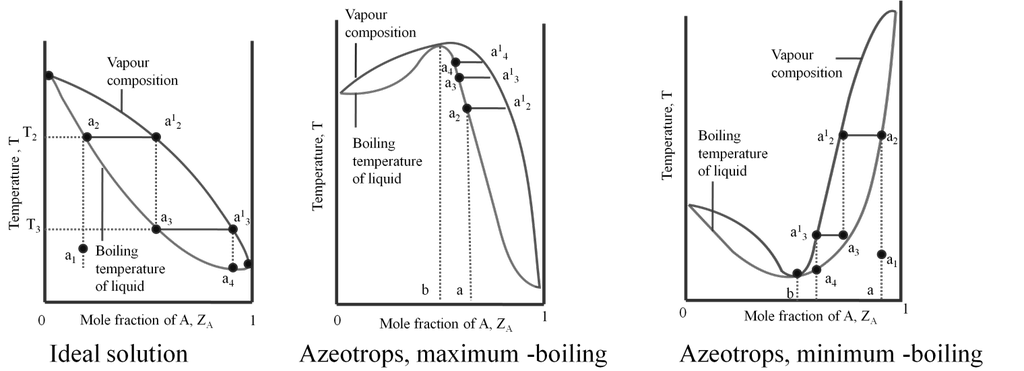
Azeotropes are binary solutions (liquid mixtures) having the same composition in liquid and vapour phase and it is not possible to separate the components of an azeotrope by fractional distillation.
Types of azeotropes:
(a) Minimum boiling azeotrope: The non- ideal solutions showing large positive deviation form minimum boiling azeotrope at a specific composition. Example; 95% ethanol and 5% water (by volume) Ethanol = 351.3K, Water = 373 K, Azeotrope = 351.1K
(b) Maximum boiling azeotrope. The non- ideal solutions showing large negative deviation form maximum boiling azeotrope at a specific composition. Example : 68% Nitric acid and 32% water (by mass) Nitric acid = 359K , Water = 373 K, Azeotrope = 393.5K

 Kaysons Publication
Kaysons Publication
