- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
RESONANCE
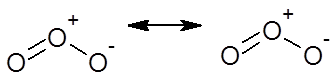
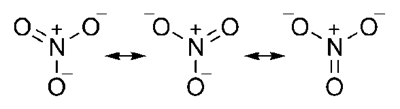
(1) Whenever a molecule can be represented by two or more structure that are different only in arrangement of electrons – i.e. they have some arrangement of atoms (both structural and stereo) there is Resonance
(2) When these structures are of about same stability (i.e. have same energy content), then resonance is important.
(3) The actual molecular is a hybrid of all there structures and cannot be satisfactorily explained by any are of them. Each structure contributes to the hybrid.
(4) The actual structure cannot be drawn as per Lewis structure and the lewis structures is not actual molecule.
(5) The resonance hybrid is more stable than any of the contributing structures.
(6) The contributing structures do not exist at all.
(7) The contributing structures are called canonical forms.
Ozone molecule
Carbon monoxide molecule
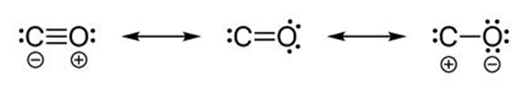
Nitrate ion
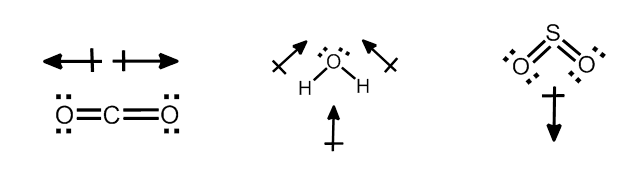
POLARITY IN COVALENT BONDS
When covalent bond is formed between two similar atoms, for example in H2, O2, or F2, the shared pair of electrons is equally attracted by the two nuclei. The electron pair is situated exactly between the two identical nuclei. The bond so formed is called nonpolar covalent bond.
In case of a hetero-nuclear molecule like HCl, the shared electron pair between the two atoms gets displaced more towards chlorine as E.N. of fluorine is far greater than that of hydrogen. The resultant covalent bond is a polar covalent bond
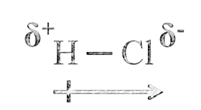
Dipole Moment has a Magnitude and a Direction
Dipoles:

 Kaysons Publication
Kaysons Publication
