- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Reaction Mechanism
A mechanism for a reaction is a collection of elementary processes (also called elementary steps or elementary reactions) that explains how the overall reaction proceeds.
A mechanism is a proposal from which you can work out a rate law that agrees with the observed rate laws. The fact that a mechanism explains the experimental results is not a proof that the mechanism is correct. A mechanism is our rationalization of a chemical reaction, and devising mechanism is an excellent academic exercise in itself.
Basic Definitions:-
1° Carbon → Carbon attached to only one Carbon
2° Carbon → Carbon attached to only two Carbon
3° Carbon → Carbon attached to only three Carbon
4° Carbon → Carbon attached to only four Carbon
1° Hydrogen → Hydrogen attached to only 1° Carbon
2° Hydrogen → Hydrogen attached to only 2° Carbon
3° Hydrogen → Hydrogen attached to only 3° Carbon
In CH4 the C is called super 1°
Some other common terms
![]()
(CH2 = CH –) Vinylic
(CH2 = CH – CH2 –) Allylic
![]()
Example 1:-
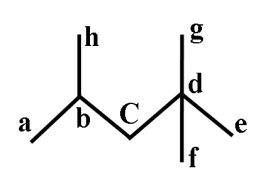
1°C → a, e, f, g, h
2°C → c
3°C → b
4°C → d
Example 2:-
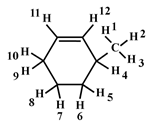 1°Hyd → 1, 2, 3
1°Hyd → 1, 2, 3
2°Hyd → 5, 6, 7, 8
3°Allylic → 4
2°Allylic → 9, 10
2°Vinylic → 11, 12
Introduction
A chemical equation is a symbolic representation of a chemical. Reaction it indicates the initial reactants and final products involved in a change. Reactants generally consist of two species:
Substrate+Reagent →Product
1. One which is being attacked; it is called a substrate.
2. Other which attacks the substrate; it is referred to as a reagent. These two interact to form products.
Types of Reagents
Reagents are attacking species and are created by breaking of covalent bonds.
A covalent bond can get cleaved either by:-
(i)Heterolytic Cleavage
(ii) Homolytic Cleavage
(i):- heterolytic cleavage, the bond breaks in such a fashion that the shared pair of electrons remains with the more electronegative atom . After heterolysis, one atom has a sextet electronic structure and a positive charge and the other, a valence octet with at least one lone pair and a negative charge. Thus, heterolytic cleavage of bromomethane will happen as follows as shown below.
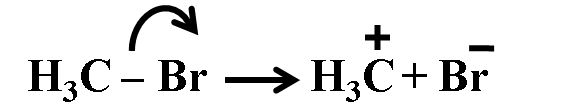
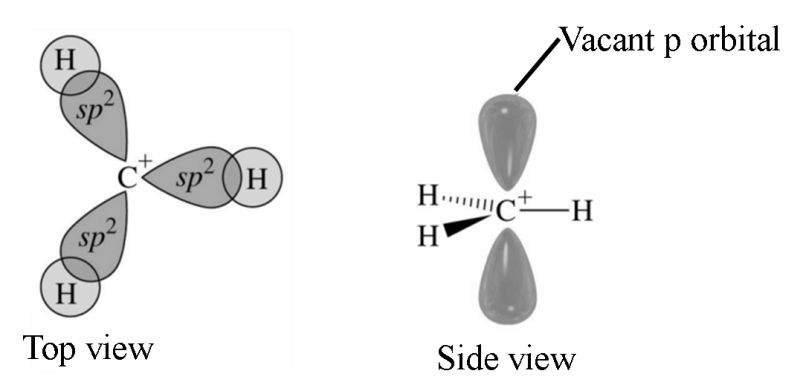
Carbocations
A species having a carbon atom possessing positive charge is called a carbocation (earlier called Carbonium ion). Carbocations are highly unstable and reactive species. Alkyl groups directly attached to the positively charged carbon stabilize the carbocations due to inductive and hyperconjugations effects.
The observed order of carbocation stability is:-
benzylic ~ 3° > Allylic ~ 2° > 1° > CH3+>Vinylic
These carbocations have trigonal planar shape with positively charged carbon being sp2 hybridised. The remaining carbon orbital is perpendicular to the molecular plane and contains no electrons.
Carbanions
The heterolytic cleavage can also give a species in which carbon gets the shared pair of electrons. Such a carbon species carrying a negative charge on carbon atom is called carbanion. Carbanions are also unstable and reactive species. The carbanion exists in a sp3 hybridisation and trigonal pyramidal geometry.
The observed order of carbanion stability is: ![]() > 1° > 2° > 3°
> 1° > 2° > 3°
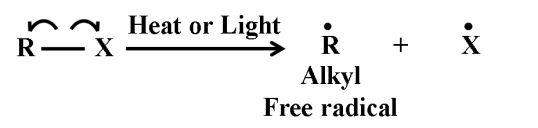
(ii) In Homolytic cleavage, one of the electrons of the shared pair in a covalent bond goes with each of the bonded atoms. Thus, in homolytic cleavage, the movement of a single electron takes place instead of an electron pair. The single electron movement is shown by ‘half-headed’ curved arrow. Such cleavage results in the formation of neutral species (atom or group) which contains an unpaired electron. These species are called free radicals. Like carbocations and carbanions, free radicals are also very reactive. A homolytic cleavage can be shown as:-
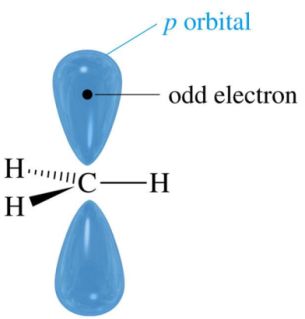
Carbon free radicals are also Sp2 hybridized as shown in figure.
The observed order of free radical stability is: Benzylic ~ Allylic > 3° > 2° > 1° > CH3
Nucleophiles and Electrophiles
A reagent that has an electron pair is called a nucleophile (Nu:) i.e., nucleus seeking and the reaction is then called nucleophilic. A reagent that takes away an electron pair is called electrophile (E+) i.e., electron seeking and the reaction is called electrophilic.
During a polar organic reaction, a nucleophile attacks an electron deficient (relatively positive charge) centre of the substrate. Nucleophile may be neutral or negatively charge. All nucleophiles are Lewis base. Similarly, the electrophiles attack electron rich centre of the substrate. Electrophile may be neutral or positively charged. All electrophile are Lewis acids.
Examples of electrophiles:-

 Kaysons Publication
Kaysons Publication
