- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
IONIZATION ENERGY
• If sufficient energy is provided, the attraction between the outer electron and the nucleus can be overcome and the electron will be removed from the atom
FIRST IONIZATION ENERGY (IE1)
• The minimum amount of energy required to remove the most loosely bound electron from an isolated gaseous atom to form a 1+ ion
Na (g) + 496 KJ/ Mol → Na+ (g) + e-
• Second ionization energy (IE2)
The minimum amount of energy required to remove the 2nd electron from a gaseous 1+ ion
IE1: Ca(g) + 590 kJ/mol ® Ca+(g) + e-
IE2: Ca+(g) + 1145 kJ/mol ® Ca2+(g) + e-
The 2nd electron "feels" higher nuclear charge (stronger attractive force) since the electron-electron repulsion has been decreased: For all atoms IE2 > IE1
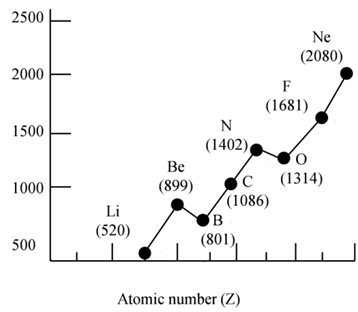
Ionization Energy: Trends
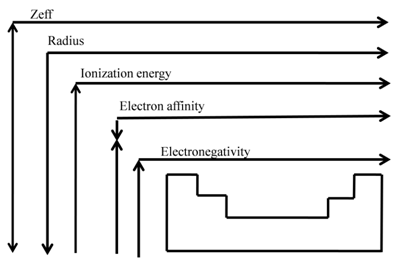
• IE1 increases from left to right along a period since the effective nuclear charge (Zeff) "felt" by the outermost electrons increases
- There are some exceptions to this general trend caused by additional stability of filled and half- filled subshells (orbitals with the same )
- IE1 decreases as we go down a group since the outermost electrons are farther from the nucleus
IONIZATION ENERGY: PERIODICITY
IMPORTANT CONCLUSIONS
- Atoms of noble gases have completely filled outer shell, the smallest radii among the elements in the same period, and the highest ionization energies
- Atoms of metals, especially those to the left in the periodic chart, ionize easily forming cations and attaining the electron configuration of noble gases
- Atoms of non-metals, especially those to the right in the periodic chart, are very unlikely to loose electrons easily - their ionization energies are high
ELECTRON AFFINITY
- For most non-metals, it is much easier to achieve the stable electron configuration of a noble gas by gaining rather than losing electrons
- Therefore, non-metals tend to form anions
- Electron affinity is a measure of an atom's ability to form negative ions
- The amount of energy released when an electron is added to an isolated gaseous atom to form an ion with a -1 charge.
Cl(g) + e- ® Cl-(g) + 349 kJ/mol
Sign conventions for electron affinity
• If electron affinity < 0 (energy is released)
• If electron affinity > 0 (energy is absorbed)
Compare cation- and anion-forming processes:
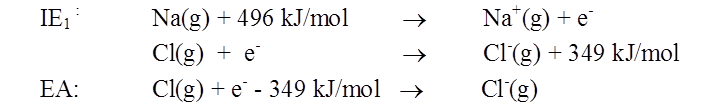
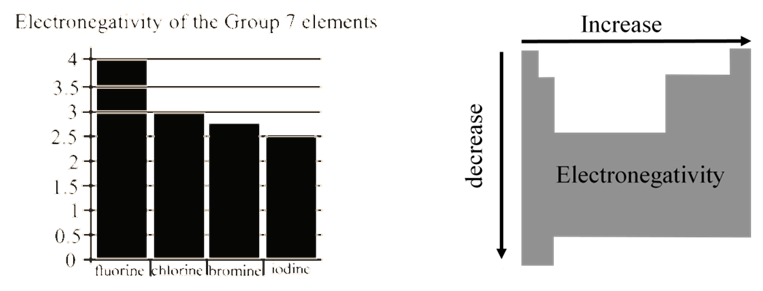
ELECTRON AFFINITY: TRENDS
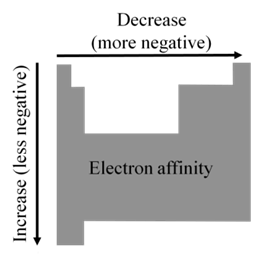
EA becomes more negative on going from left to right along a period
• There are some exceptions to this general trend caused by
additional stability of filled and half- filled subshells
(orbitals with the same l )
• EA becomes less negative as we go down a group because
the attraction of the outermost electrons to the nucleus weakens
ELECTRON AFFINITY: PERIODICITY
• Important conclusions
- Noble gases have completely filled outer shell and therefore zero electron affinity
- Non-metals, especially halogens, gain electrons easily forming anions and attaining the electron configuration of noble gases
- Metals are usually quite unlikely to gain electrons and form anions
Electron Gain Enthalpies (kJ mol-1) of group17 elements
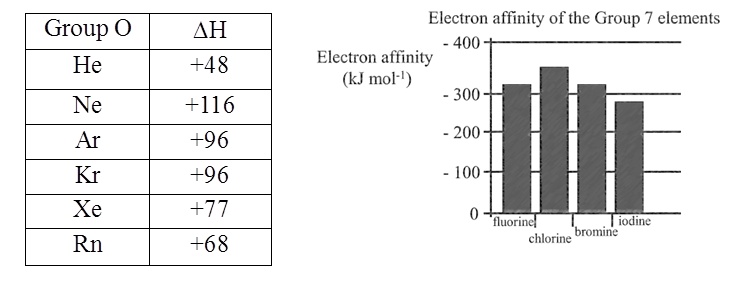
ELECTRONEGATIVITY
• Measures the tendency of an atom to attract electrons when chemically combined with another element
• If element "likes" electrons - high electronegativity (electronegative element)
• If element "dislikes" electrons - low electronegativity (electropositive element)
Sounds like the electron affinity but different
• Electron affinity measures the degree of attraction of an electron by a single atom forming an anion
• Electronegativity measures the attraction of electrons to the atom in chemical compounds
- The scale for electronegativity was suggested by Linus Pauling
- It is a semi-qualitative scale based on data collected from studying many compounds
ANOMALOUS PROPERTIES OF SECOND PERIOD ELEMENTS
The first element of each of the groups 1 (lithium) and 2 (beryllium) and groups 13-17 (boron to fluorine) differs in many respects from the other members of their respective group. Behaviour of lithium and beryllium is more similar with the second element of the following group i.e., magnesium and aluminium, respectively. This sort of similarity is commonly referred to as diagonal relationship in the periodic properties. What are the reasons for the different chemical behaviour of the first member of a group of elements in the s- and p-blocks compared to that of the subsequent members in the same group? The anomalous behaviour is attributed to their small size, large charge/ radius ratio, high Electronegativity and absence of d orbital

 Kaysons Publication
Kaysons Publication
