- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
INTRODUCTION
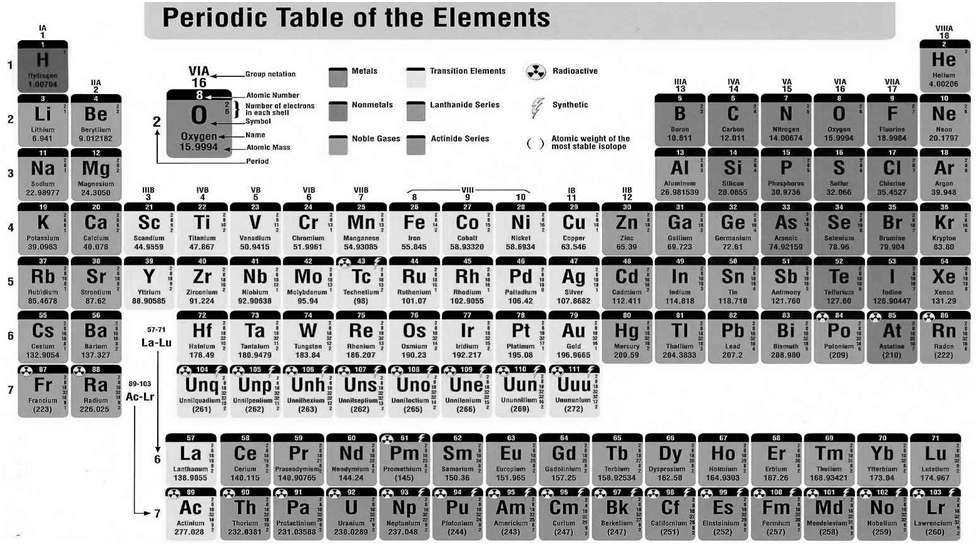
In 1800, only 31 elements were known. By 1865, the number of identified elements had more than doubled to 63. At present 114 elements are known.
With such a large number of elements it is very difficult to study individually. the chemistry of all these elements is also difficult to handle individually.
LAW OF TRIADS
Johann Döbereiner classified elements in group of three elements called triads.
In Döbereiner triad the atomic weight of the middle element is very close to the arithmetic mean of the other two elements
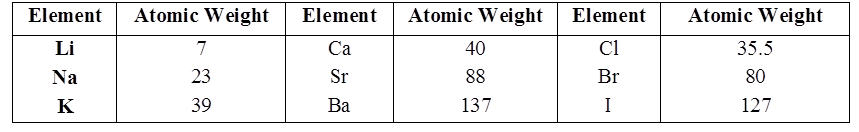
LAW OF OCTAVES
Since Döbereiner Law of triads worked only for few elements, it was dismissed.
Chancourtois arranged elements in order of increasing atomic weights and made a cylindrical table of elements.
John Newland arranged the elements in the increasing order of atomic weight and noted that the properties of the every eighth element are similar to the first one. This relationship is called as “Law of octaves”
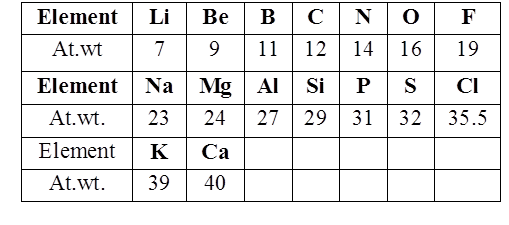
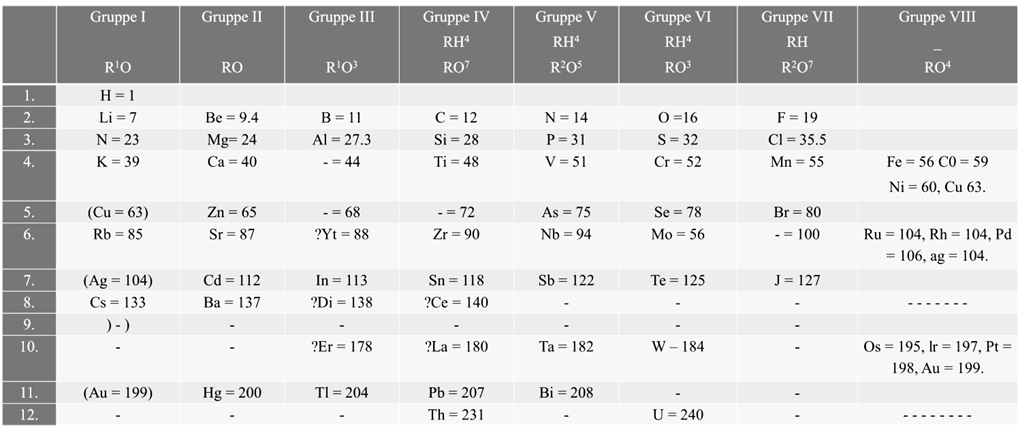
MENDELEEV’S PERIODIC TABLE
According to Mendeleev’s periodic law the physical and chemical properties of elements are periodic functions of their atomic weights.
Merits of Mendeleev’s periodic table:
- Mendeleev’s periodic table was very helpful in remembering and studying the properties of large number of elements
- Mendeleev’s periodic table helped in correcting the atomic masses of some of the elements like gold, beryllium and platinum based on their positions in the periodic table
- Mendeleev could predict the properties of some undiscovered elements like scandium, gallium and germanium. By this intuition, he had left gaps for the undiscovered elements while arranging elements in his periodic table
SUCCESS OF MENDELEEV’S WORK
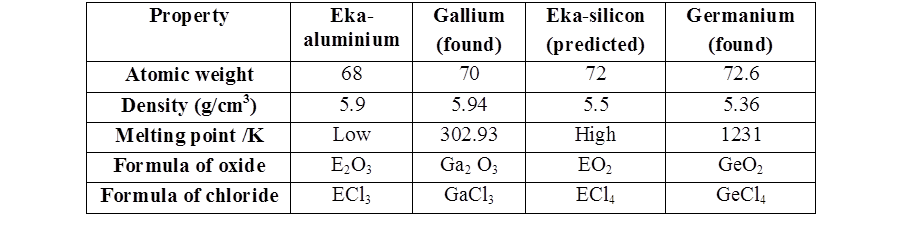
Limitation of Mendeleev’s table
- Position of hydrogen is not correctly defined in periodic table. It is placed in group I though it resembles both group 1 and 17.
- In certain pairs of elements increasing order of atomic masses was not obeyed. For example argon (Ar, atomic mass 39.9) is placed before potassium (K, atomic mass 39.1)
- Isotopes were not given separate places in the periodic table although Mendeleev's classification is based on the atomic masses.
- Some similar elements are separated and dissimilar elements are grouped together. For example copper and mercury resembled in their properties but had been placed in different groups. On the other hand lithium and copper were placed together although their properties are quite different.
- Mendeleev did not explain the cause of periodicity among the elements.
- Lanthanoids and actinoids were not given a separate position in the table.
Modern Periodic Table
Henry Moseley showed that the atomic number is a more fundamental property of an element than its atomic mass.
Mendeleev’s Periodic Law was, therefore, accordingly modified.
Modern Periodic Law:
The physical and chemical properties of the elements are periodic functions of their atomic numbers
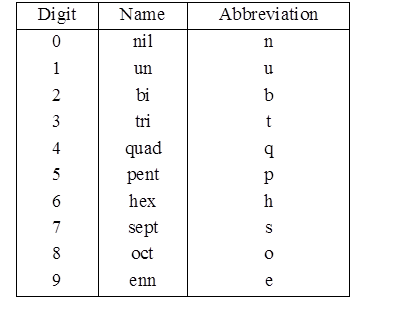
Nomenclature of elements of Atomic mass > 100
Electronic Configurations for Periods and groups
Electronic configuration of Zirconium (A. No. 40): 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d2
Iodine I53 = 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5
We can classify the elements into four blocks viz.,
s-block, p-block, d-block and f-block depending on the type of atomic orbitals that are being filled with electrons.
Hydrogen and Helium are exception to this rule
The s-Block Elements
The elements of Group 1 (alkali metals) and Group 2 (alkaline earth metals) which have ns1 and ns2 outermost electronic configuration belong to the s-Block Elements.
The p-Block Elements
The outermost electronic configuration varies from ns2np1 to ns2np6 in each period. The p-Block Elements comprise those belonging to Group 13 to 18 and these together with the s-Block Elements are called the Representative Elements or Main Group Elements.
The d-Block Elements (Transition Elements)
These elements have the general outer electronic configuration (n-1)d1-10ns0-2 . These are the elements of Group 3 to 12 in the centre of the Periodic Table. These are characterised by the filling of inner d orbitals by electrons and are therefore referred to as d-Block Elements. They are all metals.
The f-Block Elements (Inner-Transition Elements)
They have outer electronic configuration (n-2)f1-14 (n-1)d0-1ns2
The two rows of elements at the bottom of the Periodic Table, called the Lanthanoids, Ce(Z = 58) to Lu(Z = 71) and Actinoids, Th(Z = 90) to Lr (Z = 103) .
METALS, NON METALS AND METALLOIDS
Metals comprise more than 78% of all known elements and appear on the left side of the Periodic Table. Metals are usually solids at room temperature [mercury is an exception; gallium and caesium also have very low melting points (303K and 302K, respectively)]. Metals usually have high melting and boiling points. They are good conductors of heat and electricity. They are malleable (can be flattened into thin sheets by hammering) and ductile (can be drawn into wires). In contrast, non-metals are located at the top right hand side of the Periodic Table. In fact, in a horizontal row, the property of elements change from metallic on the left to non-metallic on the right. Non-metals are usually solids or gases at room temperature with low melting and boiling points (boron and carbon are exceptions). They are poor conductors of heat and electricity.
Most non-metallic solids are brittle and are neither malleable nor ductile. The elements become more metallic as we go down a group; the non-metallic character increases as one goes from left to right across the Periodic Table. The change from metallic to non-metallic character is not abrupt as shown by the thick zigzag line in periodic table.
The elements (e.g., silicon, germanium, arsenic, antimony and tellurium) bordering this line and running diagonally across the Periodic Table show properties that are characteristic of both metals and non-metals. These elements are called Semi-metals or Metalloids

 Kaysons Publication
Kaysons Publication
