- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
HYBRIDISATION AND SHAPE
Salient features of hybridisation: The main features of hybridisation are as under:
1. The number of hybrid orbitals is equal to the number of the atomic orbitals that get hybridised.
2. The hybridised orbitals are always equivalent in energy and shape
3. The hybrid orbitals are more effective in forming stable bonds than the pure atomic orbitals.
4. These hybrid orbitals are directed in space in some preferred direction to have minimum repulsion between electron pairs and thus a stable arrangement.
Therefore, the type of hybridisation indicates the geometry of the molecules
Important conditions for hybridisation
(i) The orbitals present in the valence shell of the atom are hybridised.
(ii) The orbitals undergoing hybridisation should have almost equal energy.
(iii) Promotion of electron is not essential condition prior to hybridisation.
(iv) It is not necessary that only half filled orbitals participate in hybridisation. In some cases, even filled orbitals of valence shell take part in hybridisation
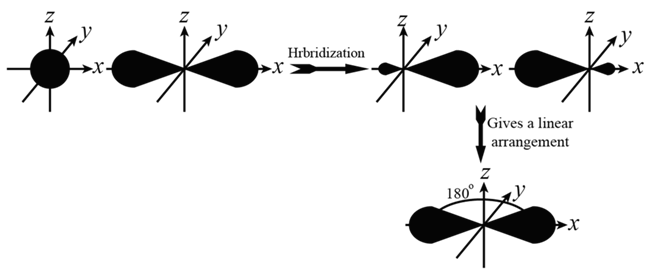
(i) sp HYBRIDISATION:
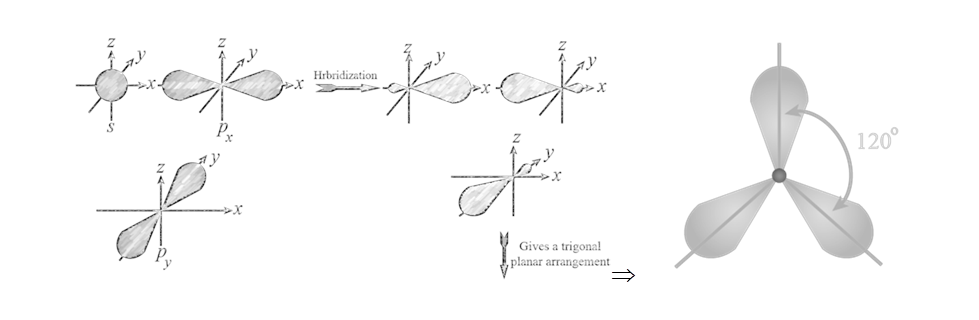
(II) sp2 hybridisation:
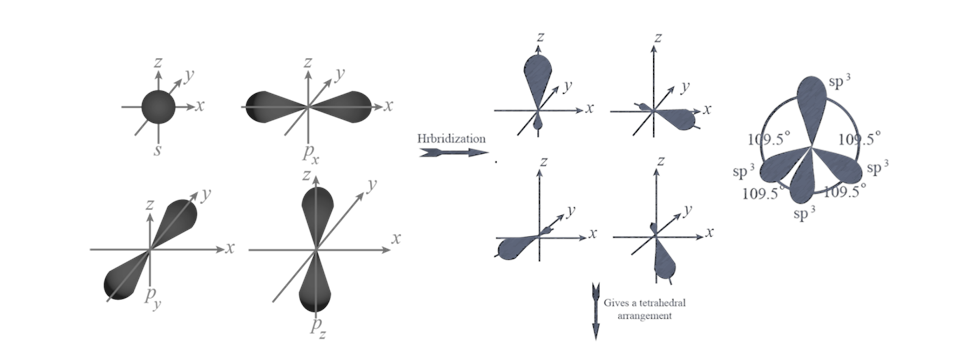
(iii) sp3 HYBRIDISATION
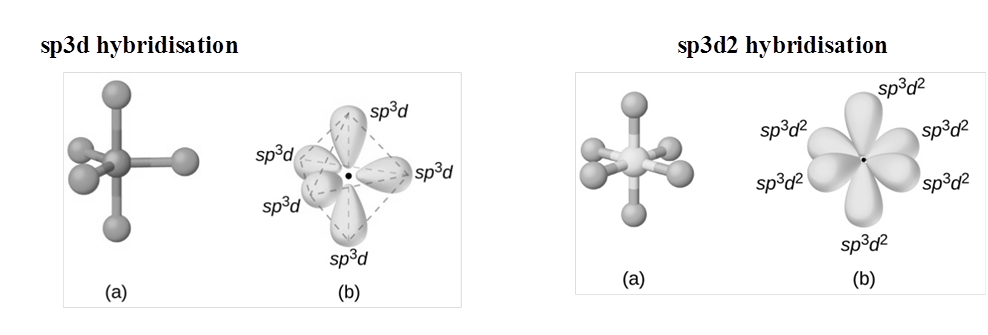
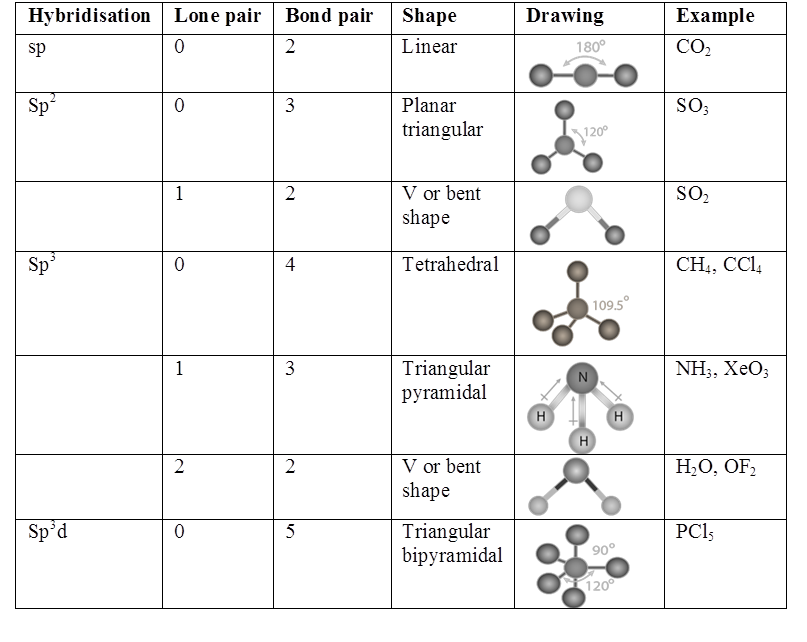
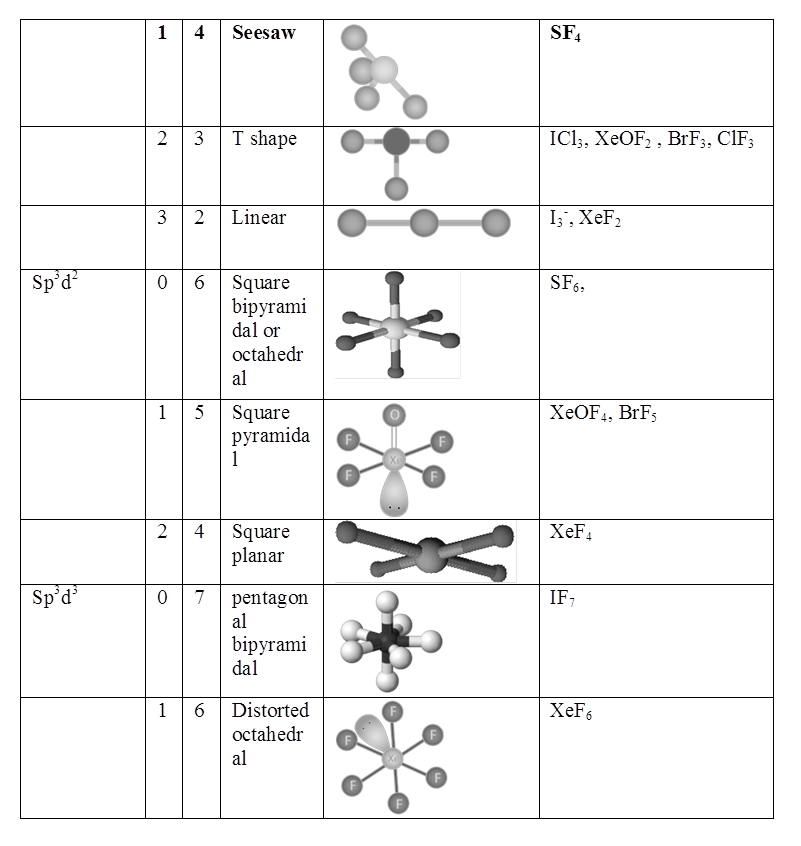
RULES TO FIND HYBRIZATION AND SHAPE (HOMONUCLEAR MOLECULAR)
- Draw the Lewis structure count σ and π bonds.
- Write the electronic configuration of central atom.
- Match no. of unpaired electrons with total bonds. If it does match, then to step 5
- If it does not match drawn the E. C. of atom in exited states to match total number of bonds.
- All orbital in valence shell having unpaired e- & lone pairs undergo hybridization (π)- electrons do not hybridize.
- Shape is not hybridization. For shape only bond pairs are counted not lone pairs.
- The actual bond angle may be different then hybridization angle because of following.
(a) Repulsion lp – lp > lp – bp > bp – bp
(b) Difference in E. N. of atoms in the bond.

 Kaysons Publication
Kaysons Publication
