Lewis structure
- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
INTRODUCTION
The attractive force which holds together the constituent particles (atoms, ions or molecules) in chemical species is known as chemical bond.
- Kössel-Lewis Approach to Chemical Bonding
They assumed that atom have positive kernel surrounded by electrons occupying the corners of a cube. If they have all the eight electrons in their outer shell they will be stable (octet rule). Otherwise they achieve stability (octet) through chemical bonding.
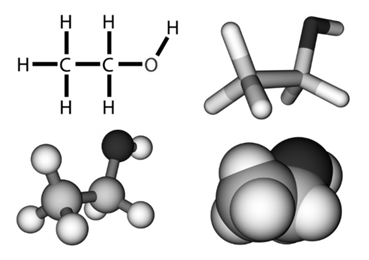
LEWIS STRUCTURE
Why chemical bonds are formed.
If the resultant molecule has lower Gibb's energy then the reacting species, then chemical bonds are formed.
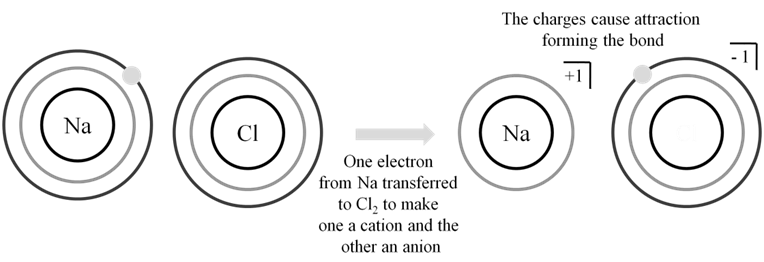
IONIC BOND
Ionic bonds are strong electrostatic forces between cation & anion which are formed when an atom looses an electron or gains an electron.
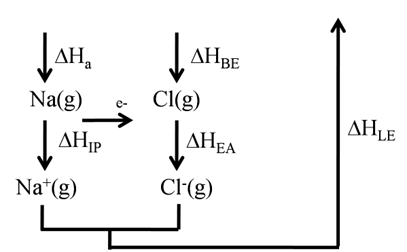
BORN HABER CYCLE FOR SODIUM CHLORIDE
![]()
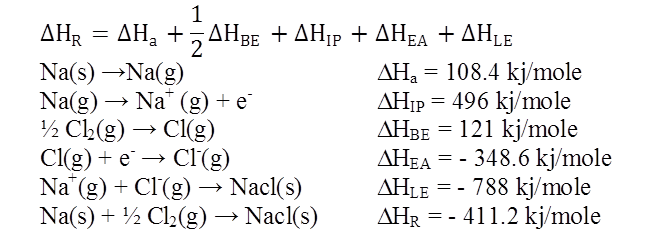
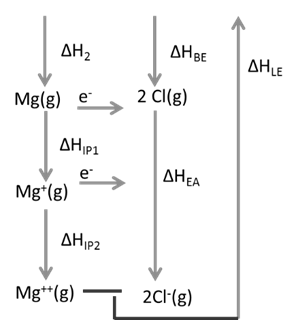
Ex.1: Draw Born Haber cycle for formation of Magnesium chloride
![]()
![]()
PROPERTIES OF IONIC COMPOUNDS
- They are crystalline in nature
- They have high Melting and Boiling point
- Hard and Brittle
- Soluble in polar solvents
- Conduct electricity in molten state and aqueous state but not in solid state.
- Do not show Isomerism.
VARIABLE ELECTROVALENCY
Fe (26) → 3s2 3p6 3d6 4s2
Fe+2 (24) → 3s2 3p6 3d6 (less stable)
Fe+3 (23) → 3s2 3p6 3d5 (more stable)
Solubility of ionic compounds in water. There are two things happen on dissolving ionic compounds in water.
1. Breaking of ionic lattice
2. Mixing of ions in water
For the first process, lattice energy to be provided to the solution and for second process hydration energy will be released by the system
If ΔHhyd >ΔHL.E. Compound is soluble in water
(a) Lattice Energy
1. If depends on size of cation and anion.
Smaller the size greater is the Lattice Energy.
Ex. → LiCl > NaCl > KCl
→ NaCl > NaBr > NaI
2. If depends on Charge of cation and anion.
Bigger the charge, lesser is it Lattice energy.
Ex. MgCl2 > NaCl
3. In case anions are extremely large, then the rule one charges smaller the cation lesser is Lattice Energy.
Ex. MgSO4 < CaSO4 < SrSO3 < BaSO4
(b) Hydrogen energy
Smaller the cation, greater is the hydration enthalpy.
Ex. Compare Solubility BaSO4 and CaSO4
L.E. Þ BaSO4 > CaSO4
Hyd. Energy Þ CaSO4 > BaSO4
So CaSO4 is more soluble.
Ex. Compare solubility of NaCl and BaCl2
L.E. Þ BaCl2 > NaCl
Hyd. Energy Þ NaCl > BaCl2
So NaCl is more soluble.
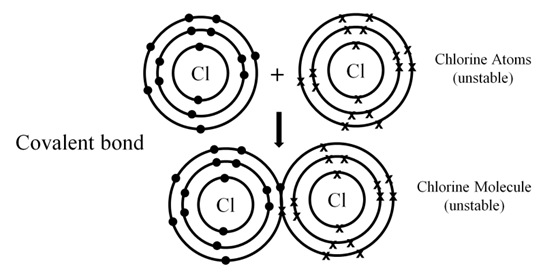
COVALENT BOND
Combining of unpaired electrons of atoms to achieve a stable configuration and formation of molecules is called covalent bond.
SIGMA (s) BOND: THIS TYPE OF COVALENT BOND is formed by the end to end (head-on) overlap of bonding orbitals along the inter-nuclear axis. This is called as head on overlap or axial overlap
s - s overlapping: In this case, there is overlap of two half killed s-orbitals along the inter-nuclear axis as shown below:

s-p overlapping: This type of overlap occurs between half filled s-orbitals of one atom and half filled p-orbitals of another atom:
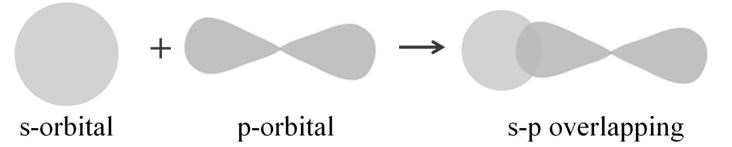
p-p overlapping: This type of overlap takes place between half filled p-orbitals of the two approaching atoms:
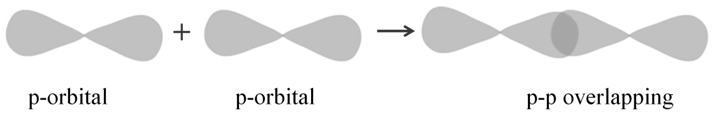
Pi (π) BOND: IN THE FORMATION OF π BOND
The atomic orbitals overlap in such a way that their axes remain parallel to each other and perpendicular to the inter-nuclear axis.
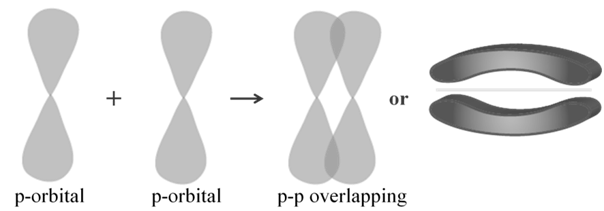
Characteristic of Convent compounds
- Physical state ® gases, liquids of law b.p. & soft solids
- Melting Point/Boiling point ® with exception of network solids, they have low M.P. and B.P.
- Electrical conductance ® generally bad conductors
- Solubility ® soluble in non-polar solvents
- Isomerism ® yes Physical state ® gases, liquids of law b.p. & soft solids
- Melting Point/Boiling point ® with exception of network solids, they have low MP/B.
- Electrical conduce ® generally bed conductors
- Solubility ® soluble in non-polar solvents
- Isomerises ® yes
COMPARISON BETWEEN IONIC AND COVALENT BONDS

COMPARISON BETWEEN IONIC AND COVALENT COMPOUNDS

Some representative Bond Energies and Bond Lengths
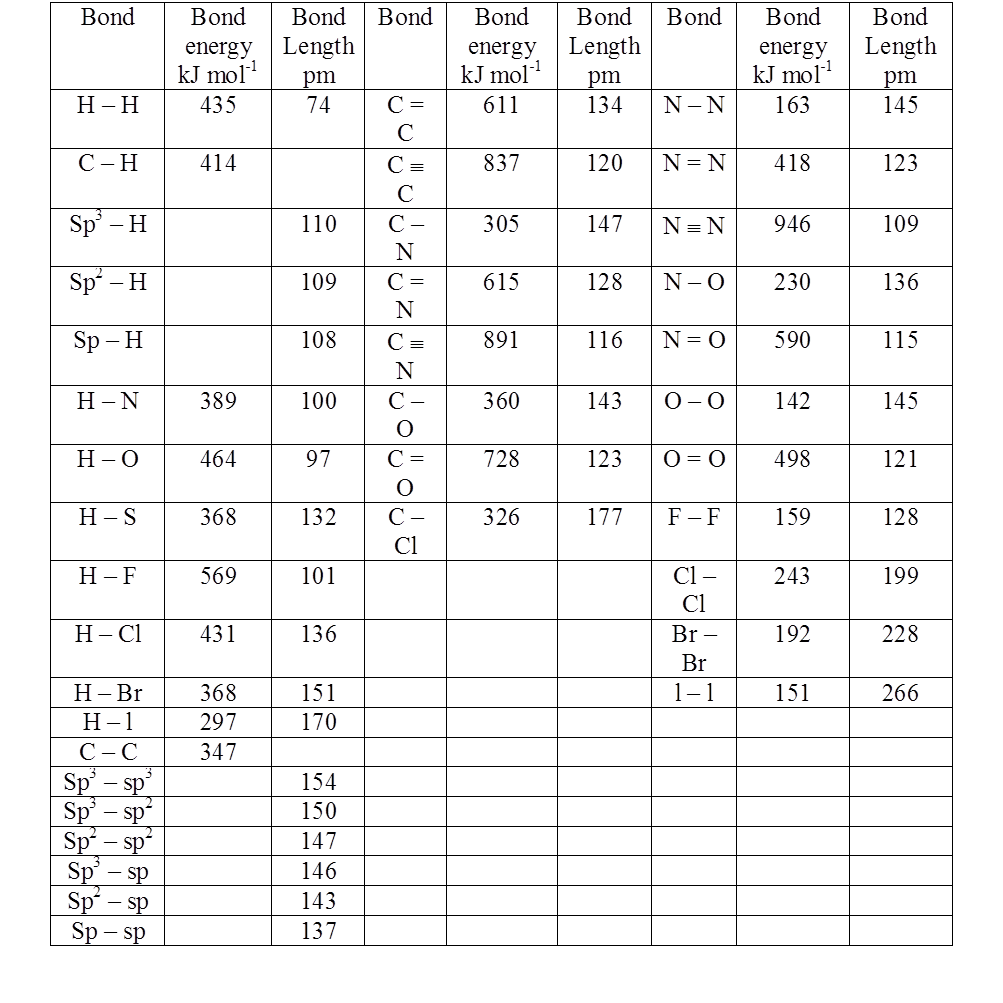
Coordinate Bond
- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
COORDINATE BOND
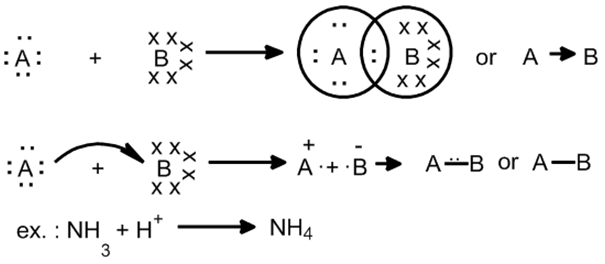
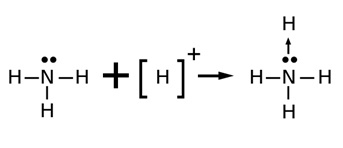
Dative bond or semi-polar bond.
METALLIC BOND
The bonding which holds metals atoms firmly together on account of force & attraction between metal cation and mobile sea electrons is called metallic bonding.
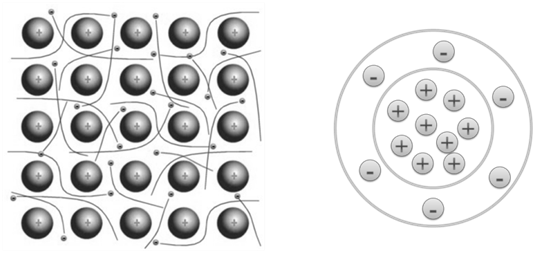
PROPERTIES OF METALS & NON METALS

LANDON FORCES AND DISPERSION FORCES
London forces between two Helium atoms

DIPOLE - DIPOLE FORCES
Dipole-dipole forces act between the molecules possessing permanent dipole. Ends of the dipoles possess partial charges, and these charges are shown by Greek letter delta (δ). Partial charges are always less than the unit electronic charge. The polar molecules interact with neighboring molecules.
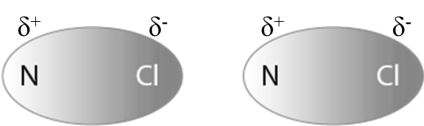
Dipole-dipole interaction energy between stationary polar molecules (as in solids) is proportional to ![]() , Where r is the distance between polar molecules.
, Where r is the distance between polar molecules.
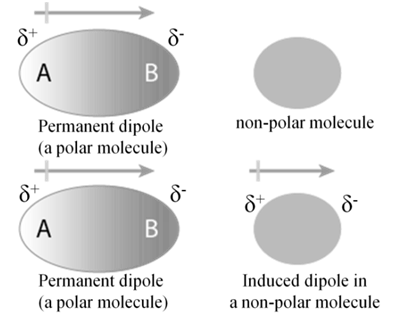
DIPOLE INDUCED DIPOLE FORCES
This type of attractive forces operate between the polar molecules having permanent dipole and the molecules lacking permanent dipole.
Permanent dipole of the polar molecule induces dipole on the electrically neutral molecule by deforming its electronic cloud
Hydrogen Bond
- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
HYDROGEN BOND
This is special case of dipole-dipole interaction.. This is found in the molecules in which highly polar N-H, O-H or H-F bonds are present. Although hydrogen bonding is regarded as being limited to N, O and F; but species such as Chlorine may also participate in hydrogen bonding. Energy of hydrogen bond varies between 10 to 100 kJ mol.
Hydrogen bond can be classified in three types
1. Inter molecular H bond. Same molecule
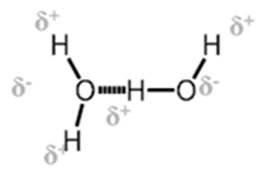
- Inter molecular H bond. Different molecule
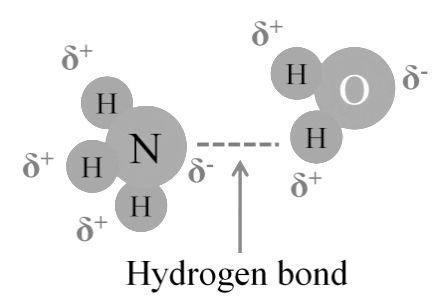
Hydrogen Bonding between Ammonia and Water
- Intra Molecular Hyrogen Bond
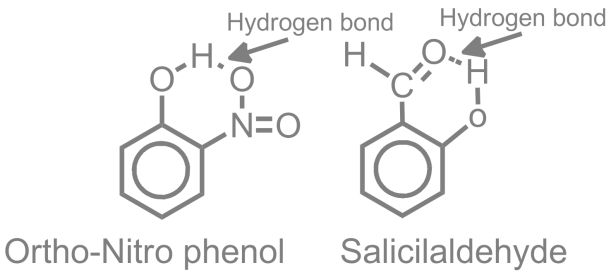
HYDROGEN BOND STRENGTH
Hydrogen bonds can vary in strength from weak (1–2 kJ mol−1) to strong (161.5 kJ mol−1 in the ion Typical enthalpies in vapour include:
F − H···: F (161.5 kJ/mol or 38.6 kcal/mol), illustrated uniquely by HF2−,
O − H···: N (29 kJ/mol or 6.9 kcal/mol), illustrated water-ammonia
O − H···: O (21 kJ/mol or 5.0 kcal/mol), illustrated water-water, and alcohol-alcohol
N − H···: N (13 kJ/mol or 3.1 kcal/mol), illustrated by ammonia-ammonia
N − H···: O (8 kJ/mol or 1.9 kcal/mol), illustrated water-amide
Bond Length
It is defined as the angle between the orbitals containing bonding electron pairs around the central atom in a molecule/complex ion. Bond angle is always determined experimentally
A. Ionic Compound:- bond length is sum of cationic radius and anionic radius.
B. Covalent compound:
The bond length is measured by spectroscopic, x-ray diffraction and electron diffraction. Covalent radius is then calculated from this.
Lewis structure:- unable to give bond angle
VESPR they:- much better in predicate bond angle
M. O. T:- Give bond angle much better
Bond Energy or Enthalpy
It is defined as the amount of energy required to break one mole of bonds of a particular type between two atoms in a gaseous state.
For a diatomic molecular Bond energy simply energy required to break the bond.
![]()
![]()
For Hetero-nuclear molecular it is average energy or mean energy.
Ex. H2O(g) → H(g) + OH(g) ∆H = 502
OH(g) → H(g) + O(g) ∆H = 427
![]()
Bond length or bond distance is the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of the molecule
.![]()
1. Sigma bond (s )> Pie (p) bond
2. Triple bond > double bond > single bond
3. s – s overlap > s – p overlap > p – p overlap
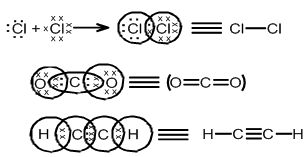
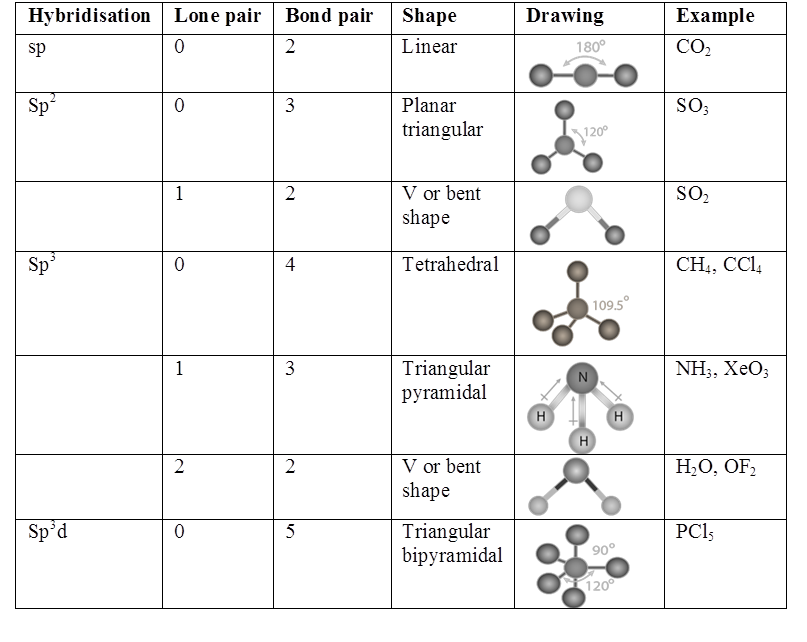
OCTET RULE
Kössel and Lewis in 1916 developed an important theory of chemical combination between atoms known as electronic theory of chemical bonding. According to this, atoms can combine either by transfer of valence electrons from one atom to another (gaining or losing) or by sharing of valence electrons in order to have an eight electrons in their valence shells.
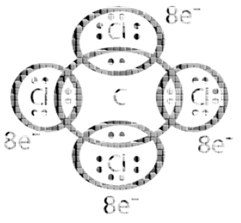
Exceptions to the Octet Rule:
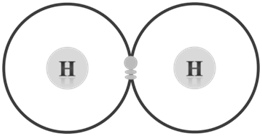
- Hydrogen molecule: Hydrogen has one electron in its first energy shell (n = 1). It needs only one more electron to fill this shell, because the first shell cannot have more than two electrons. This configuration (1s2) is similar to that of noble gas helium and is stable. In this case, therefore, octet is not needed to achieve a stable configuration
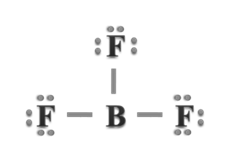
Incomplete octet of the central atom: The octet rule cannot explain the formation of certain molecules of lithium, beryllium, boron, aluminium, etc. (LiCl, BeH2, BeCl2, BH3, BF3) in which the central atom has less than eight electrons in the valence shell as shown below:
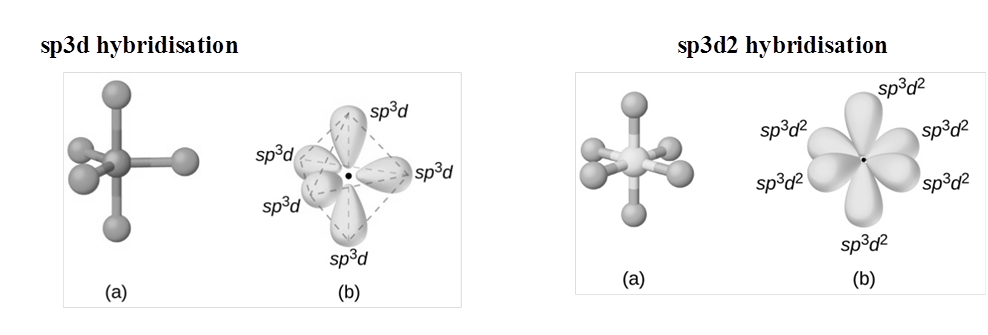
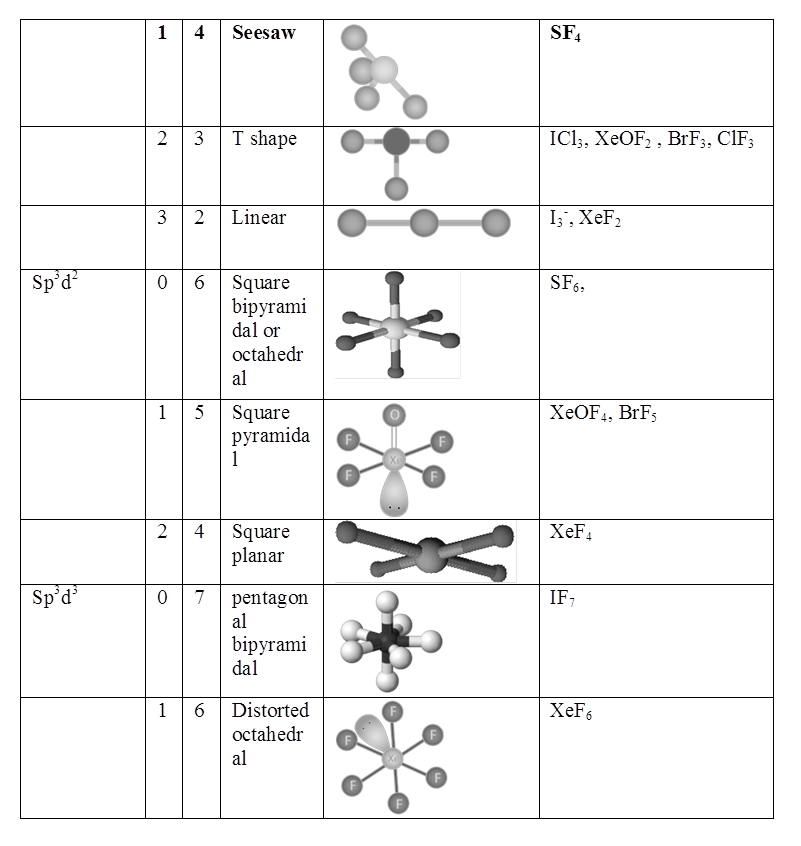
Expanded octet of the central atom: There are many stable molecules which have more than eight electrons in their valence shells. For example, PF5, has ten; SF6 has twelve and IF7 ha fourteen electrons around the central atoms, P, S, and I respectively
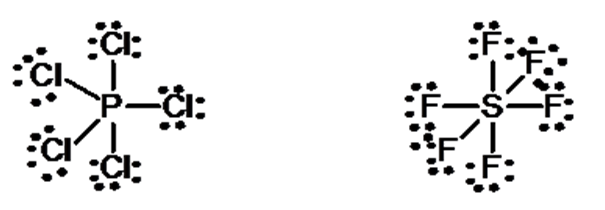
Odd electron molecules: There are certain molecules which have odd number of electrons, like nitric oxide, NO and Nitrogen dioxide, NO2. In these cases, octet rule is not satisfied for all the atoms.

It may be noted that the octet rule is based upon the chemical inertness of noble gases. However, it has been found that some noble gases (especially xenon and krypton) also combine with oxygen and fluorine to form a large number of compounds such a XeF2, KrF2, XeOF2, XeOF4, XeF6, etc.
This theory does not account for the shape of the molecules. It cannot explain the relative stability of the molecule in terms of the energy.
LEWIS STRUCTURE
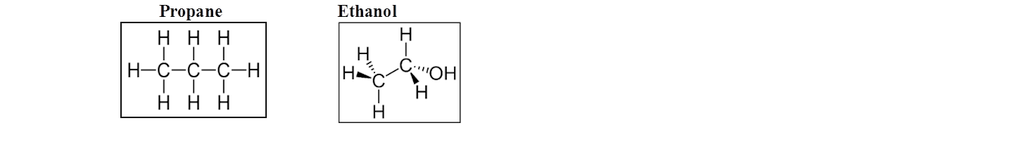
Lewis structure and bonding theory cannot explain the shape of molecule.
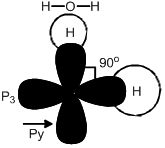
Shape of H2O.
H → 1s2
O → 1s2 2s2 px2 py1 pz1
Angle H ─ O ─ H
Is 90o as per this mode but shell L = 104.5o
Hybridisation
- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
HYBRIDISATION AND SHAPE
Salient features of hybridisation: The main features of hybridisation are as under:
1. The number of hybrid orbitals is equal to the number of the atomic orbitals that get hybridised.
2. The hybridised orbitals are always equivalent in energy and shape
3. The hybrid orbitals are more effective in forming stable bonds than the pure atomic orbitals.
4. These hybrid orbitals are directed in space in some preferred direction to have minimum repulsion between electron pairs and thus a stable arrangement.
Therefore, the type of hybridisation indicates the geometry of the molecules
Important conditions for hybridisation
(i) The orbitals present in the valence shell of the atom are hybridised.
(ii) The orbitals undergoing hybridisation should have almost equal energy.
(iii) Promotion of electron is not essential condition prior to hybridisation.
(iv) It is not necessary that only half filled orbitals participate in hybridisation. In some cases, even filled orbitals of valence shell take part in hybridisation
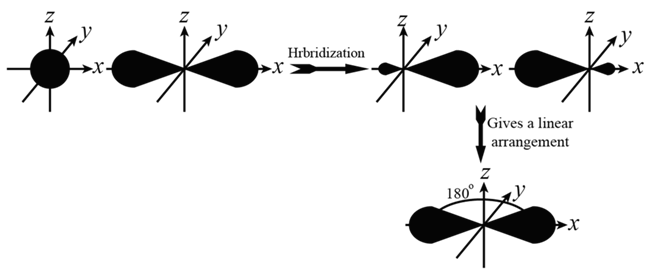
(i) sp HYBRIDISATION:
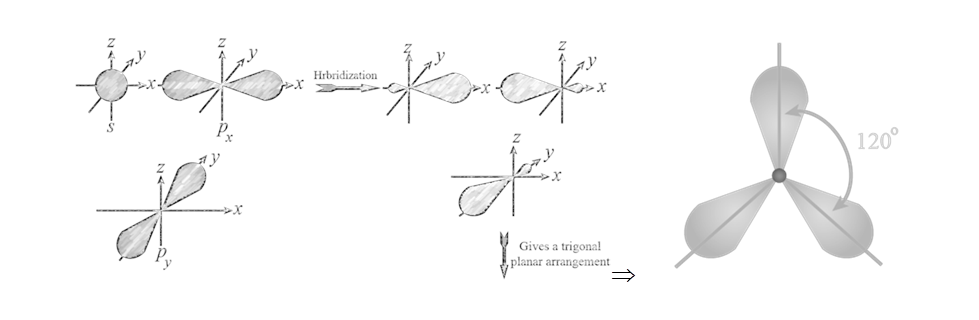
(II) sp2 hybridisation:
(iii) sp3 HYBRIDISATION
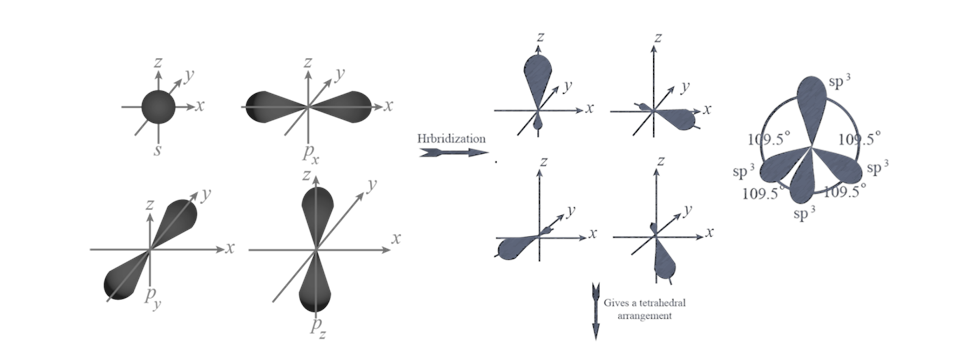
RULES TO FIND HYBRIZATION AND SHAPE (HOMONUCLEAR MOLECULAR)
- Draw the Lewis structure count σ and π bonds.
- Write the electronic configuration of central atom.
- Match no. of unpaired electrons with total bonds. If it does match, then to step 5
- If it does not match drawn the E. C. of atom in exited states to match total number of bonds.
- All orbital in valence shell having unpaired e- & lone pairs undergo hybridization (π)- electrons do not hybridize.
- Shape is not hybridization. For shape only bond pairs are counted not lone pairs.
- The actual bond angle may be different then hybridization angle because of following.
(a) Repulsion lp – lp > lp – bp > bp – bp
(b) Difference in E. N. of atoms in the bond.
Resonance
- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
RESONANCE
(1) Whenever a molecule can be represented by two or more structure that are different only in arrangement of electrons – i.e. they have some arrangement of atoms (both structural and stereo) there is Resonance
(2) When these structures are of about same stability (i.e. have same energy content), then resonance is important.
(3) The actual molecular is a hybrid of all there structures and cannot be satisfactorily explained by any are of them. Each structure contributes to the hybrid.
(4) The actual structure cannot be drawn as per Lewis structure and the lewis structures is not actual molecule.
(5) The resonance hybrid is more stable than any of the contributing structures.
(6) The contributing structures do not exist at all.
(7) The contributing structures are called canonical forms.
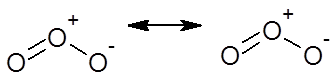
Ozone molecule
Carbon monoxide molecule
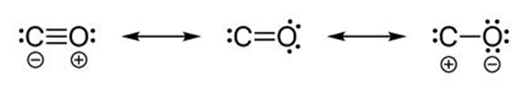
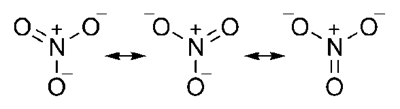
Nitrate ion
POLARITY IN COVALENT BONDS
When covalent bond is formed between two similar atoms, for example in H2, O2, or F2, the shared pair of electrons is equally attracted by the two nuclei. The electron pair is situated exactly between the two identical nuclei. The bond so formed is called nonpolar covalent bond.
In case of a hetero-nuclear molecule like HCl, the shared electron pair between the two atoms gets displaced more towards chlorine as E.N. of fluorine is far greater than that of hydrogen. The resultant covalent bond is a polar covalent bond
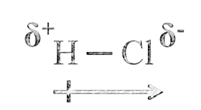
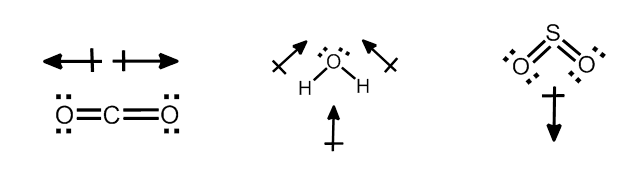
Dipole Moment has a Magnitude and a Direction
Dipoles:
Molecular orbital theory
- Books Name
- Kaysons Academy Chemistry Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Limitation of VSEPR theory
- It cannot explain why oxygen molecule is paramagnetic
- It cannot explain formation of H2+ molecule. And may more
MOLECULAR ORBITAL THEORY (MOT)
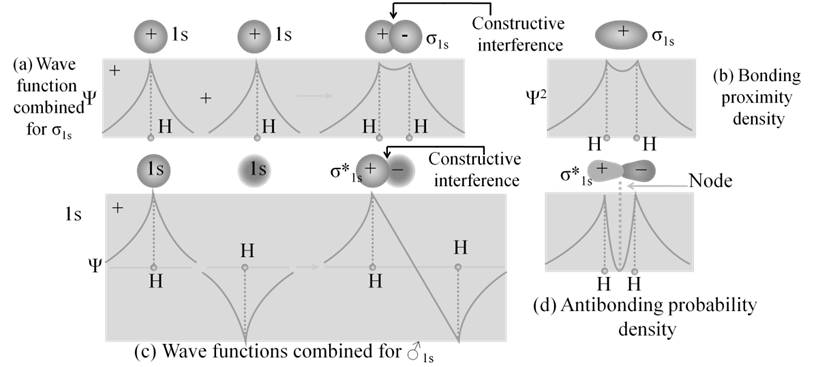
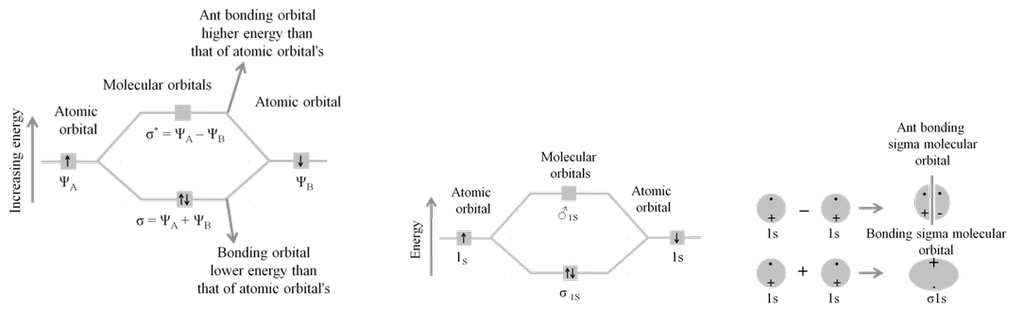
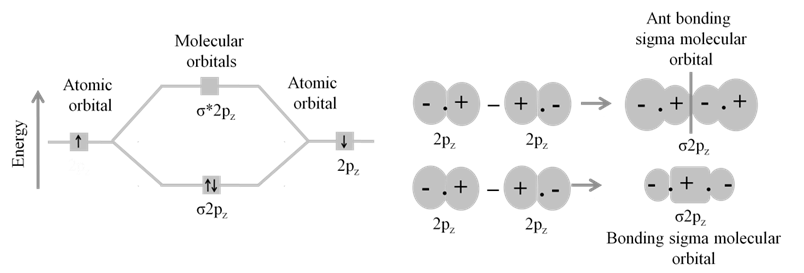
· Basic idea of MOT is that atomic orbitals of individual atoms combine to form molecular orbitals. Electrons in molecule are present in the molecular orbitals which are associated with several nuclei.
· The molecular orbital formed by the addition of atomic orbitals is called the bonding molecular orbital ( s ).
· The molecular orbital formed by the subtraction of atomic orbital is called antibonding molecular orbital (s *).
· The sigma (s ) molecular orbitals are symmetrical around the bond-axis while pi (p ) molecular orbitals are not symmetrical.
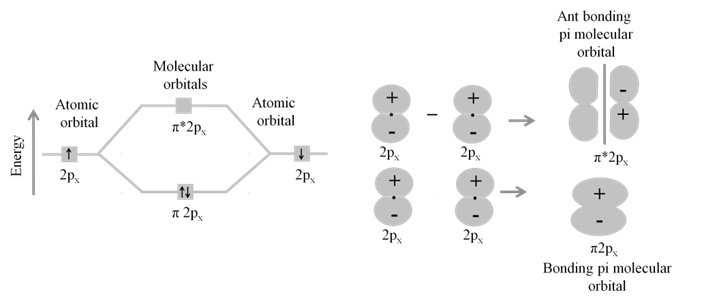
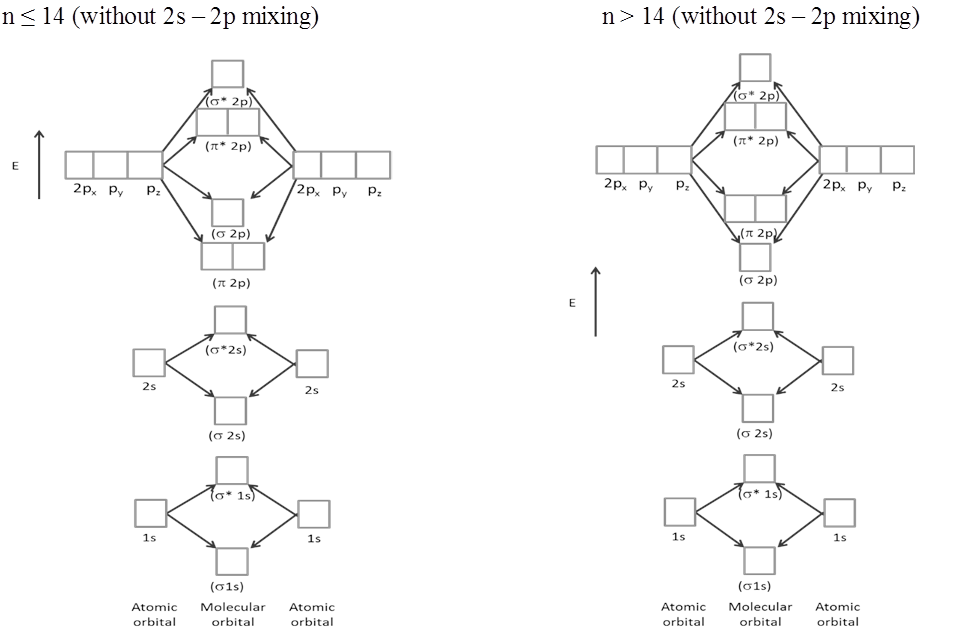
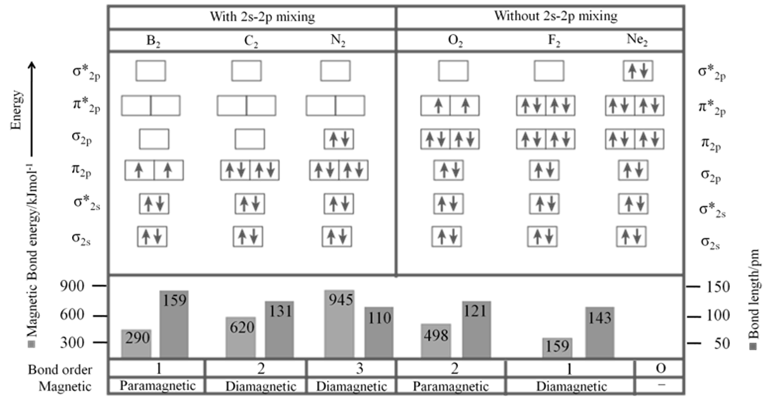
- Sequence of energy levels of molecular orbitals changes for diatomic molecules like Li2, Be2, B2, C2, N2 is (For n £ 14) ; s1s < s*1s < s2s < s*2s < p2py = p2pZ < s2px < p*2py = p*2pZ < s*2px
- Sequence of energy levels of molecular orbitals changes for diatomic molecules like O2, F2, Ne2 ( for n >14) is s1s < s*1s < s2s < s*2s < s2px < p2py = p2pZ < p*2py = p*2pZ < s*2px
- Bond order (b.o.) is defined as one half the differences between the number of electrons present in the bonding and the antibonding orbitals.
- Bond Order =
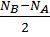
NB = Bonding electrons NA = anti-bonding electrons

 Kaysons Publication
Kaysons Publication
