Air, Water, Soil.
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Introduction
→ Life on earth depends on resources like soil, water, air and energy from sun.
→ Uneven heating of air over land and water-bodies causes winds.
→ Evaporation of water from water-bodies and subsequent condensation give us rain.
→ Pollution of air, water and soil affect the quality of life.
→ We need to conserve our natural resources and use them in a sustainable manner.
→Various nutrients are used again and again in a cycle fashion. This leads to a certain balance
between the various components of the biosphere.
Natural Resources
→ The resources available on the earth and the energy from the sun are necessary to meet the
basic requirements of all life forms on the earth.
→ The stocks of nature which are useful to mankind are known as natural resources.
Examples: air, water, soil, minerals etc.
Resources on the earth
→ The outermost crust of the earth is called the lithosphere.
→ Water covers 75% of the earth’s surface. It is also found underground. These comprise the
hydrosphere.
→ The air that covers the whole of the earth like blanket is called the atmosphere.
Biosphere
→ All living things on earth together with atmosphere, the hydrosphere and the lithosphere intera
and make life possible is known as biosphere.
• It may be:
(i) Biotic components: Plants and animals.
(ii) Abiotic components: Air, water and soil.
Water, oxygen, carbon and nitrogen.
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Biogeochemical Cycles
→ The flow of substances from non-living to living and back to non-living is called the cycling of
substances.
→ The cycling of chemical elements like carbon, oxygen, nitrogen, phosphorus, sulphur and water
the biosphere is called biogeochemical cycle.
→ It operates through soil, water, air and biotic factors.
→ The whole process in which water evaporates and falls on the land as rain and later flows back
into the sea via rivers is known as water cycle.
→ When sun shines, water evaporates continuously from the water bodies and forms water vapour
This water vapour rises up and goes into the atmosphere.
→ The plants absorb water from the soil and use it during the process of photosynthesis.
→ They also loose water by the process of transpiration.
→ The water vapour produced by transpiration also goes into the atmosphere.
→ The process of respiration and evaporation from the surface of animal body produces water
vapour which goes into the atmosphere.
→ The evaporation and condensation of water vapour leads to rain. During winter, the water falls
down in the form of dew or snow.
→ All of the water that falls on the land does not immediately flow back into the sea. Some of it
seeps into the soil and becomes part of the underground reservoir of fresh water.
→ The underground water is again taken by plants and water cycle continues.
oxygen Cycle
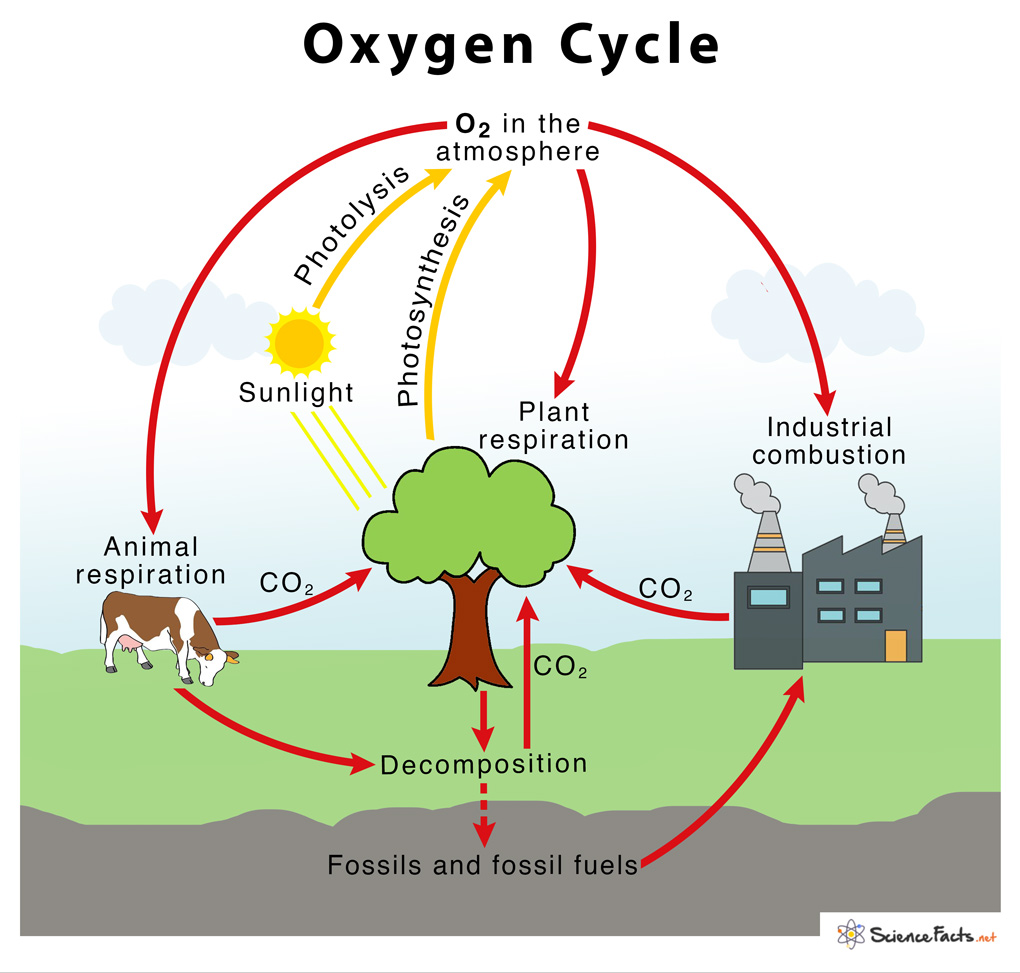
→ The percentage of oxygen in air is 21%.
→ The cyclic process by which oxygen element is circulated continuously through the living and
non-living components of the biosphere constitutes oxygen cycle.
→ Human beings and animals take oxygen from the atmosphere during the process of respiration
→ The decomposition of dead organisms also takes in oxygen from the atmosphere.
→ Respiration and decay of dead organisms release CO2 and water.
→ The carbon dioxide and water are used by the green plants during the process of photosynthesis
→ They give out oxygen during this process. This oxygen is again used by human beings and
animals.
→ Thus, the oxyen cycle keeps repeating in nature.
Carbon Cycle

→ 0.03-0.04% carbon is present in the atmosphere in the form of CO2.
→ Carbon cycle maintains the balance of the element carbon in the atmosphere. Carbon is found
various forms on the earth.
→ Carbon is present in the atmosphere as carbon dioxide.
→ Carbon can also occur as carbonates and bicarbonate salts in minerals.
→ Carbon is the essential part of nutrients like carbohydrates, fats, proteins, nucleic acids and
vitamins.
→ Carbon cycle keeps the level of CO2 constant in the atmosphere.
• The Carbon Cycle starts in plants as:
Step I: Plants use CO2 in the atmosphere, convert it into glucose in the presence of sunlight by the
process of photosynthesis. Plants and animals break these carbohydrates for energy and release
CO2 through respiration.
Step II: When the plants and animals die, fungi and bacteria decompose the dead remains. This
releases the carbon in the remains as carbon dioxide.
Step III: Some of the dead plants and animals which get buried under the earth under certain
temperature and pressure get transformed into fossil fuels like coal and petroleum.
→ On burning these fuels, CO2 is released into the atmosphere.
Nitrogen Cycle
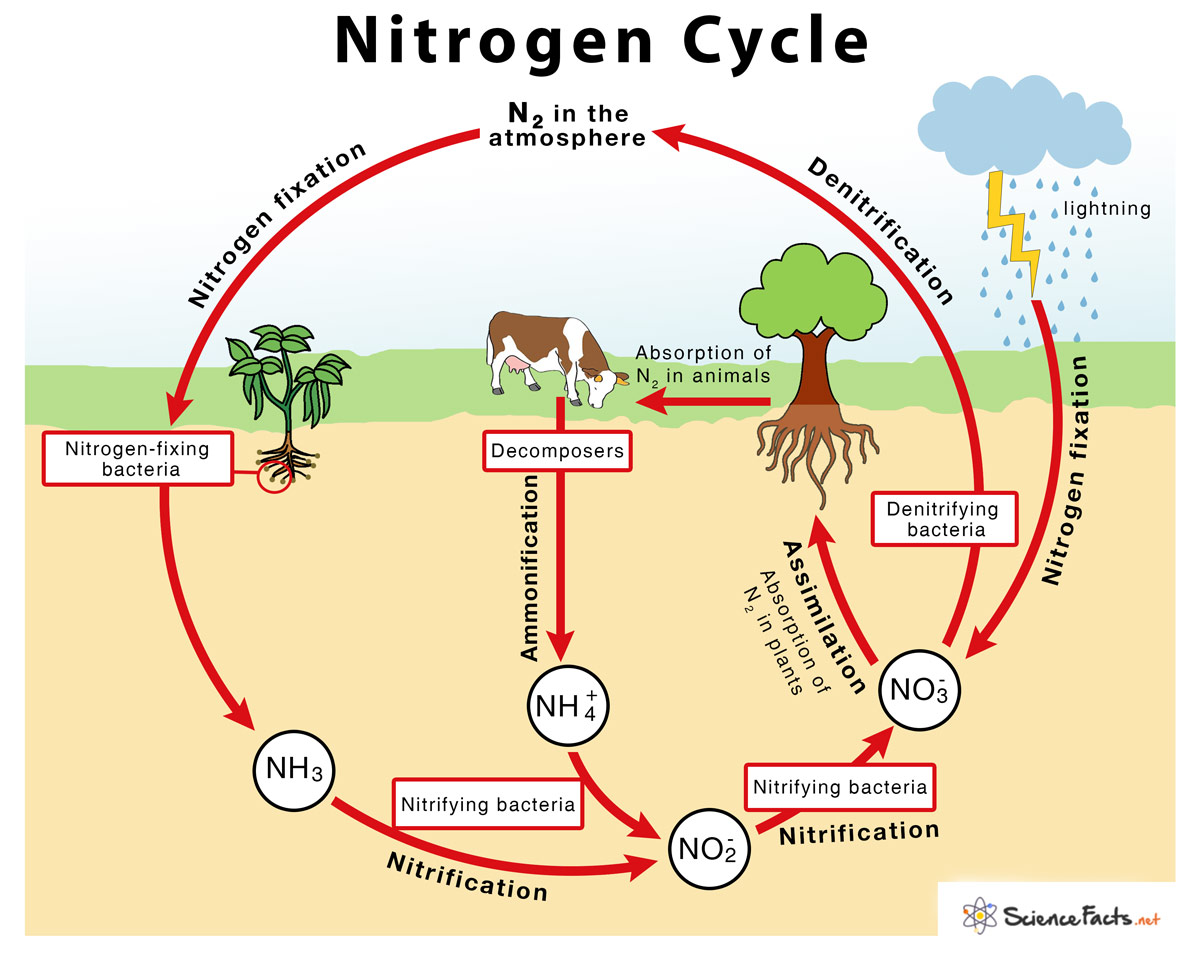
→ The sequence in which nitrogen passes from the atmosphere to the soil and organisms, and then it
is eventually released back into the atmosphere, is called nitrogen cycle.
→ Nitrogen makes up 78% of the earth’s atmosphere.
→ Nitrogen is an essential constituent of proteins, nucleic acids like DNA and RNA, vitamins and chlorophyll.
→ Plants and animals cannot utilize atmospheric nitrogen readily.
→ It has to be fixed by some organisms called nitrogen fixers.
→ Nitrogen-fixing bacteria like Rhizobium live in symbiotic association in the root nodules of certain
leguminous plants.
→ These bacteria convert atmospheric nitrogen into ammonia which is utilized readily by plants.
→ Nitrogen-fixing bacteria along with free living bacteria in the soil achieve 90% of nitrogen fixation
→ Lightning plays an important role in nitrogen fixation. When lightning occurs, the high
temperature and pressure convert nitrogen and water into nitrates and nitrites.
→ Nitrates and nitrites dissolve in water and are readily used by aquatic plants and animals.
• Ammonification: It is the process by which soil bacteria decompose dead organic matter and
release ammonia into soil.
• Nitrification: It is the process by which ammonia is converted into nitrites and nitrates.
• Denitrification: It is the process by which nitrates are converted into atmospheric nitrogen.
Air for respiration, for combustion, for moderating temperatures
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Air
→ Air is a mixture of different gases.
→ Air contains oxygen which is essential to living organisms for respiration. So it is called breath of
life.
Role of Atmosphere
→ Air is a bad conductor of heat. It keeps the average temperature of the earth constant during th
day and even during the course of the whole year.
→ Prevents the sudden increase in temperature during day time and during the night, it slows dow
the escape of heat into outer space.
Example: At moon, there is no atmosphere and so the temperature varies from 190ºC to 110ºC.
movements of air and its role in bringing rains across India.
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
The Movement of Air: Winds
→ During the day, the direction of wind is from sea to land. This is because the air above the land
gets heated faster and starts rising.
→ During the night, the direction of wind is from land to sea. This is because at night, both land an
sea start to cool.
→ The movement of air from one region to the other creates winds.
Rain
→ Rain is formed by evaporation and condensation of water through water cycle in which
distribution of water takes place.
→ Rain is very important because it carries out all the agriculture processes in the plants.
→ So we should conserve rain by contracting dams, pools etc.
Acid Rain
→ When fossil fuels are burnt, gases like sulphur dioxide and nitrogen dioxide (NO2) are released.
→ These gases are dissolving in water form nitric acid and sulphuric acid.
Air, water and soil pollution (brief introduction)
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Air
→ Air is a mixture of different gases.
→ Air contains oxygen which is essential to living organisms for respiration. So it is called breath o
life.
Air Pollution
→ An increase in the content of harmful substance (pollutants) in the air like carbon dioxide, carb
monoxide, oxides of sulphur, nitrogen, fluoride, lead, nickel, arsenic and dust particles etc. causes a
pollution.
Air pollution can cause:
• In humans: Respiratory and renal problems, high blood pressure, eye irritation, cancer.
• In plants: Reduced growth, degeneration of chlorophyll, mottling (patches/spots of colour) of
leaves.
Water: A wonder Liquid
→ The most unusual natural compound found on earth and which fulfills almost various demands
different living things.
→ About three-fourth of the earth surface is 75% are covered with water.
→ It is present underground, a very large area on the surface (sea, ocean etc.) and also in the form
of water vapour in the atmosphere.
Necessity of Water for all Organisms
→ It maintains a uniform temperature of the body.
→ All cellular processes take place in a water medium.
→ All the reactions that take place within our body and within our cells occur between substances
that are dissolved in water.
→ Water forms the habitat of many plants and animals.
Water Pollution
→ When water becomes unfit for drinking and other uses, then water is said to be polluted.
Causes of Water Pollution
→ Dumping of wastes from the industries into water bodies.
→ Washing of clothes near water bodies.
→ Spraying chemical in water field.
→ Dumping household wastes into the water bodies.
Soil
→ Soil is the portion of the earth surface consisting of disintegrated rock and decaying organic
material. It provides the support for many plants and animals.
Creation of Soil: Various Factors
• Sun
→ The sun heats up rocks during the day so that they expand. At night these rocks cool down and
contract.
→ Since all parts of the rocks do not expand and contract at the same rate, this results in the
formation of cracks and ultimately the huge rocks breaks up into smaller pieces.
• Water
→ Fast flowing water carries big and small particles of rock downstream. These rocks rub against
other rocks and the resultant abrasion causes the rocks to wear down into smaller particles.
• Wind
→ Wind carries sand from one place to another.
Living Organisms
→ Lichen (A slow growing plant)
→ Lichen, moss also grow on surface of rocks. While growing, they release certain substances tha
cause the rock surface to powder down and form a thin layer of soil.
Soil Erosion
→ Carrying away of upper fertile layer of soil by rain, wind, human activities and wrong agricultura
practice is called soil erosion.
Causes of soil erosion
→ Over grazing of land.
→ Removal of top soil by wind and water.
→ Due to lack of trees the upper layer of soil is eroded by air and water.
→ Leaving land uncultivated for long time.
Holes in ozone layer and the probable damages
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Green House Effect
→ Carbon dioxide keeps the earth warm much like glass which keeps the green house warm.
• Effect of Increase in carbon dioxide (CO2):
(i) intensifies green house effect.
(ii) leads to global warming.
(iii) increase in average temperature of earth.
(iv) may lead to melting of polar caps.
(v) sub-merging number of coastal cities.
→ Changes in environment affects us and our acitivities change the environment around us.
Environmental Problems Caused by Humans
Depletion of Ozone Layer
Ozone layer
→ Ozone layer is a protective blanket around the earth which absorbs most of the harmful UV
(ultraviolet) radiations of the sunlight, thus protecting living beings from many health hazards such
as skin cancer, cataract, destruction of plants etc.
→ Ozone (O3) layer is present at higher levels of atmosphere (i.e. stratosphere). It is a deadly poisonous at ground level.
→ Ozone layer is present in the stratosphere which is a part of our atmosphere from 16 km to 60 km
above sea level.
→ Ozone is an allotrope of oxygen. Its molecule is made up of three oxygen atoms. Molecular
formula is O3.
→ Ozone layer absorbs the ultra-violet rays coming from the sun and protects living being from
their harmful effects like skin cancer, cataract in eyes, weaken immune system.
→ The decline of ozone layer thickness in Antartica was first observed in 1985 and was termed as
ozone hole.
Reason of Ozone Depletion
→ Excessive use of CFCs (Chloro Fluoro Carbon) in refrigeratos, jet planes, spray cans, fire
extinguishers.
→ Nuclear explosion
Smog
→ Smog is a type of air pollution.
→ The word ‘smog’ comes from the blend of two words: Smoke and fog.
→ Smog can form in any climate where there is a lot of air pollution especially in cities.
Formation of ozone molecule
(i) The high energy UV radiations break down the O2 molecules into free oxygen (O) atoms.
O2 +uv → O + O (atoms)
(ii) These oxygen atoms then combine with oxygen (O2) molecule to form the ozone molecule.
O2 + O → O3 (ozone)
Depletion of ozone layer
→ The decrease in the thickness of ozone layer over Antarctica was first observed in 1985 and was
termed as ozone hole.
→ This decrease was linked to excessive use of synthetic chemicals like chlorofluorocarbons (CFC
which are used in refrigerators, ACs, fire-extinguishers, aerosols sprays etc.
→ United Nations Environment Programme (UNEP) succeeded in forging an agreement to stop CFC
production at 1986 levels (KYOTO PROTOCOL) by all countries.

 Science Made Easy
Science Made Easy
