WHAT IS SOLUTION?
A solution is a homogenous mixture of two or more substances. E.g. Nimboo pani, Soda water. A solution has a solvent and a solute as its components.
Solvent: The component of the solution that dissolves the other component in it is called the solvent.
Solute: The component of the solution that is dissolved is called the solute. in the solvent
Alloys: They are the mixtures of two or more metal or a metal and a non-metal and cannot be separated into their components by physical methods.
![]()
Properties of a Solution
• A Solution is a Homogeneous mixture.
• The particles of a solution are smaller than 1nm (10^-9 Metre) in Diameter. They cannot bean by Naked eyes.
• They are of very Small Particle Size, so they do not scatter a beam of lighting passing through the solution.
• The Solute Particles Cannot be separated from the mixture by the process of filtration.
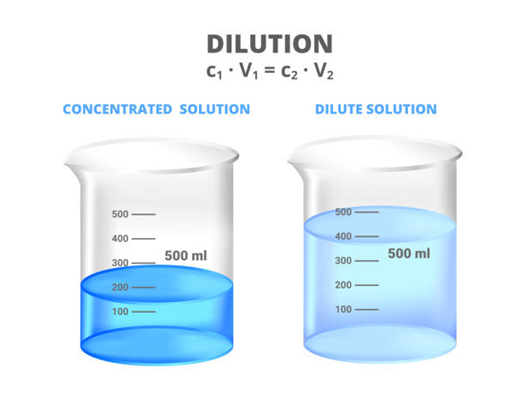
Concentration of a solution
• Saturated solution: Depending upon the amount of solute present in a solution, it can be called a dilute, concentrated or a saturated solution.
• Unsaturated solution: If the amount of solute contained in a solution is less than the saturation level, it is called an unsaturated solution.
• Solubility: The amount of the solute present in the saturated solution at this temperature is called its solubility.
• The concentration of a solution is the amount of solute present in a given amount (mass or volume) of solution, or the amount of solute dissolved in a given mass or volume of solvent.
• Concentration of solution = Amount of solute / Amount of solution
Or
Amount of solute / Amount of solvent
Ways of expressing the concentration of a solution
• Mass by mass percentage of a solution
Mass of solute / Mass of solution x100
• Mass by volume percentage of a solution
Mass of solute / Volume of solution x100

 Science Made Easy
Science Made Easy
 ACERISE INDIA
ACERISE INDIA
