CAN MATTER CHANGE ITS STATE?
Yes, they do change under various circumstances and factors which we will study below
1. Effect of change of temperature
- On increasing the temperature of solids, the kinetic energy of the particles increases.
- Due to the increase in kinetic energy, the particles start vibrating with greater speed.
- The energy supplied by heat overcomes the forces of attraction between the particles.
- The particles leave their fixed positions and start moving more freely.
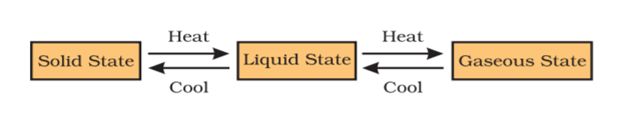
- A stage is reached when the solid melts and is converted to a liquid.
- The minimum temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point.
- The melting point of ice is 0° C (273.15 K).
- The process of melting, is also known as fusion.
- When a solid melts, its temperature remains the same
- This increase in temperature (heat) of solids is used up in changing the state by overcoming the forces of attraction between the particles without showing any rise in temperature
- This hidden heat is called latent heat
- Therefore, the amount of heat energy that is required to change 1 kg of a solid into liquid at atmospheric pressure at its melting point is known as the latent heat of fusion.
- So, particles in water at 0° C (273 K) have more energy as compared to particles in ice at the same temperature.
- Similarly, when we supply heat energy to water, particles start moving faster.
- At a certain temperature, a point is reached when the particles have enough energy to break free from the forces of attraction of each other.
- At this temperature the liquid starts changing into gas.
- The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point.
- Boiling is a bulk phenomenon i.e. each particles of the liquid gain enough energy to change into the vapour state.
- For water this temperature is 373 K (100°C = 273 + 100 = 373 K).
- The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization
- Water vapour at 373 K (100° C) have more energy than water at the same temperature. This is because particles in steam have absorbed extra energy in the form of latent heat of vaporization.
- A change of state directly from solid to gas without changing into liquid state is called sublimation. Example, vaporization of camphor
- The direct change of gas to solid without changing into liquid is called deposition. Example, soot in the chimney, making dry ice (solid carbon dioxide).
2. Effect of change of pressure
- Applying pressure and reducing temperature can liquefy gases. Examples, LPG, liquid nitrogen.

 Science Made Easy
Science Made Easy
 ACERISE INDIA
ACERISE INDIA
