The Structure of an atom
• Dalton's atomic theory suggested that the atom was indivisible and indestructible. However, the discovery of two fundamental particles in the atom the electrons and protons led to the failure of this aspect of the theory.
• Hence, J.J. Thomson was the first to propose a model for the structure of an atom.
Thomson’s Model of an Atom
According to J.J. Thomson, the structure of an atom can be compared to Christmas pudding where electrons are present inside a positive sphere.
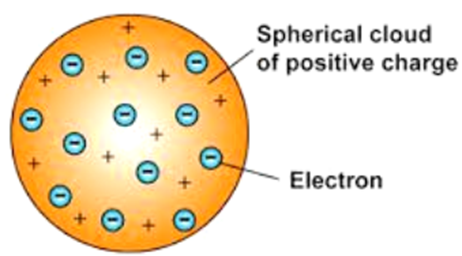
• An atom is composed of a positively charged sphere in which electrons are embedded.
• Atom is neutral as the positive and negative charged are equal in proportion.
Rutherford’s Model of an Atom
Rutherford’s Experiment
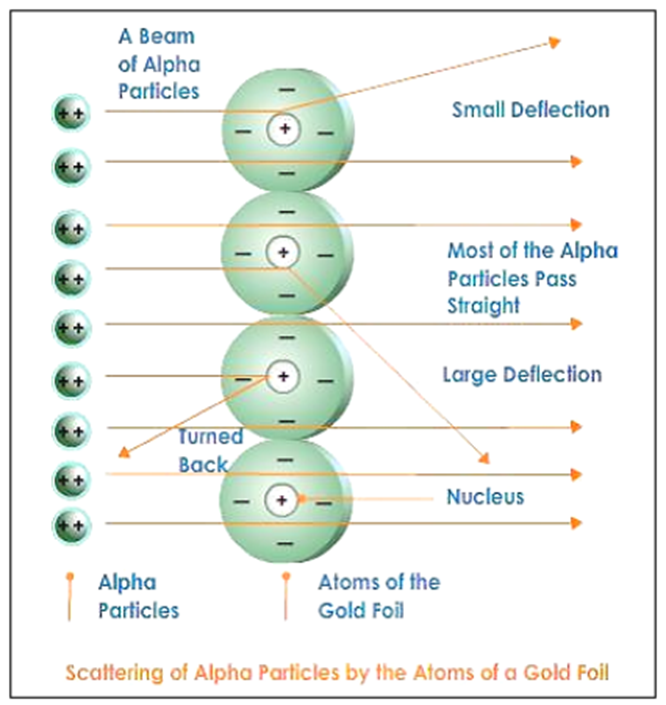
• He experimented with thin gold foil by passing alpha rays through it.
• He expected that the gold atoms will deflect the Alpha particles.
|
Observations |
Inferences |
|
Alpha particles which had high speed moved straight through the gold foil |
Atom contains a lot of empty space |
|
Some particles got diverted a by slide angles |
Positive charges in the atom are not occupying much of its space |
|
Only one out of 12000 particles bounced back |
The positive charges are concentrated over a particular area of the atom. |
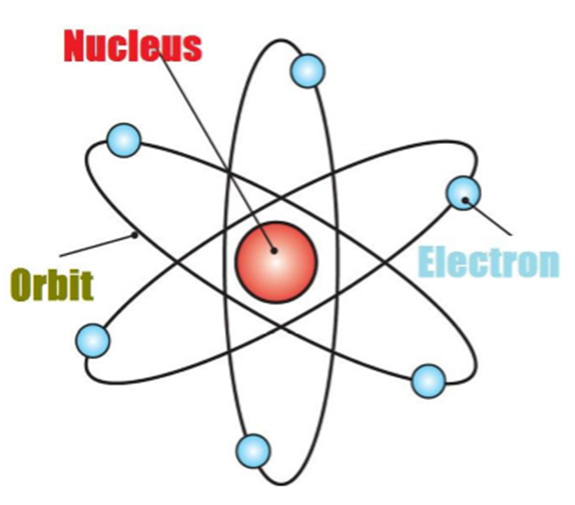
Thus, Rutherford gave the nuclear model of an atom based on his experiment which suggests that -
• Atoms contain a lot of unoccupied space
• There is a heavily positively charged substance present in the center of the atom which is called the nucleus
• The nucleus contains an equal amount of positive and negative charge.
Drawbacks of the Nuclear Model of an Atom
• The Nuclear Model of the Atom failed to explain how an atom remains stable despite having positive and negative charges present in it.
• Maxwell has suggested a theory according to which if any charged particle moves in a circular motion, it radiates energy.
• So, if electrons start moving in a circular motion around the nucleus, they would also radiate some energy which would decrease at the speed of the electrons.
• As a result, they would fall into the nucleus because of its high positive charge.
What are nucleons? – Protons and Neutrons are collectively called as Nucleons.

 Science Made Easy
Science Made Easy
 ACERISE INDIA
ACERISE INDIA
