- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Discovery of Electrons and Protons
Discovery of electrons
It was discovered by J.J. Thomson in the year 1897. He took Crooke’s tube and arranged the apparatus as shown in figure:
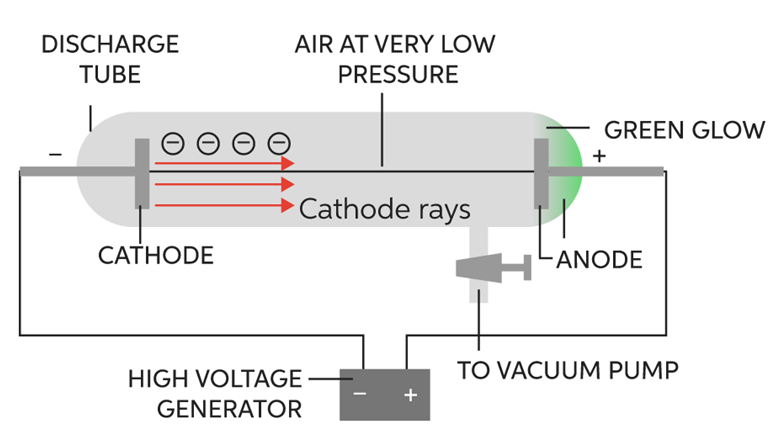
The following observations were made
1. When he passed electric current (at high voltage approximately at 10,000volts) through a gas at a pressure of 1 atm, then nothing happened as no changes were seen.
2. When he reduced the pressure to 10-2 atm, the whole tube started glowing with green colour.
3. He further reduced the pressure to 10-4 atm, the whole tube stopped glowing, but a faint green colour was still seen at the anode end.
4. To confirm, a fluorescent screen was placed at the back of the anode and anode was made perforated. When current was passed through it (in the same physical conditions), the Zinc Sulphide screen started glowing which confirmed the following fact.
The following was concluded
It proved that at these conditions, some rays were emitted through cathode and were travelling towards anode called cathode rays consisting of negatively charged particles. These particles were later called electrons. This is how the electron was discovered.
Discovery of Protons
It was discovered by E.Goldstein in the year 1920. It was actually discovered while carrying out an experiment to produce cathode rays. The apparatus used was the same:
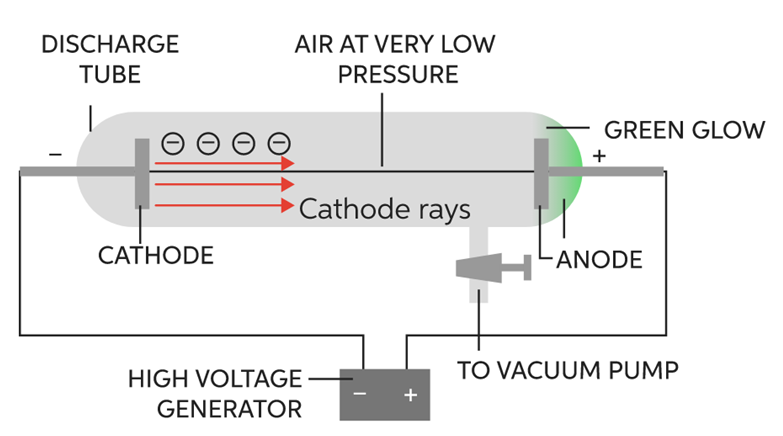
The following observations were made
It was seen that when cathode rays were produced (at high voltage and low pressure), they travelled through the gas in the discharge tube. While doing so, they ionized the gas. That is, they took electrons of gas along, leaving behind positively charged particles of the gas. These particles formed canal rays and started moving towards the cathode. These particles are called canal rays as they are not produced by anode. As these particles possess positive charge so they were named as protons.
Now after knowing about the atom, different attempts were made to know about its structure.
Thomson’s model of an atom (plum pudding model)
His full name was J.J. Thomson and he was the one who made the first attempt to explain the structure of atoms.
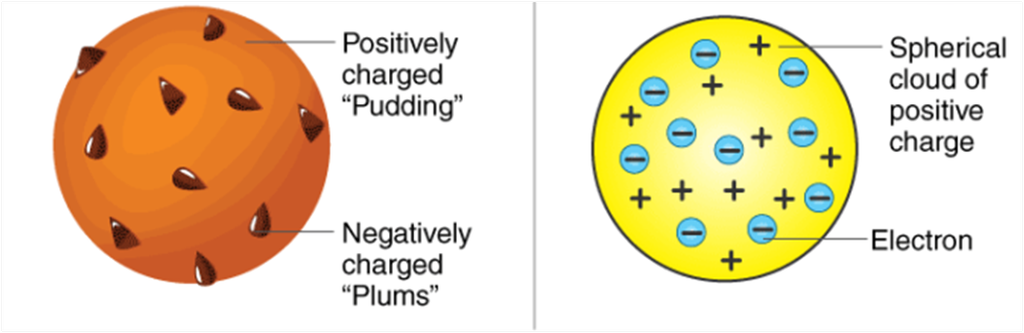
According to him, an atom is a positively charged sphere in which negative charges are present at certain places like plums in a pudding or cherries in an ice-cream. But this model was rejected as he could not explain the major point seen in his model.
Drawback of Thomson’s atomic model were as follows
He could not explain the distribution of charges and stability of an atom. As we all know that opposite charges attract each other, so, how come it is possible that few negative charges remain scattered in this big positive space. They would have been neutralized. This could not be explained by J.J. Thomson that lead to failure of his attempt.
Rutherford’s scattering experiment
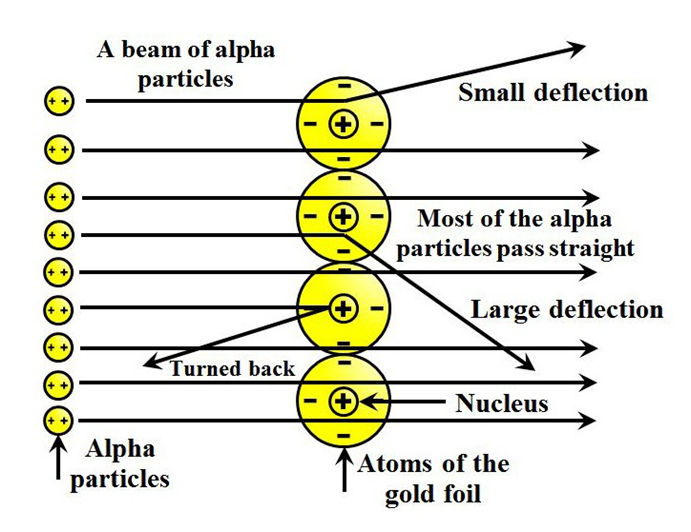
In order to understand the structure of an atom, Rutherford performed the scattering experiment. For this, he took a gold foil and passed alpha rays through it. Gold foil actually consists of many gold atoms. So, at an individual level, we are considering the observations through an atom. Alpha rays are actually positively charged rays consisting of Helium nucleus (He).
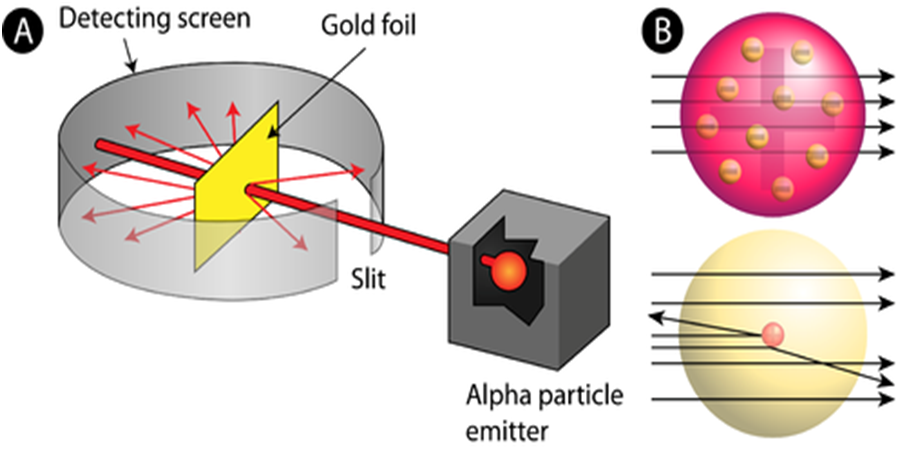
These rays, when passed through it suffered reflections at different angles and to note that a movable screen made of fluorescent material was placed around it. When the reflected rays strike that screen it causes scintillation. When he passed these rays through gold foil various observations were seen.
Observations were as follows
- Most of the rays passed straight.
- Some rays were deflected through small & large angles.
- Some rays rebound back.
The following conclusions were made
- Most of the space in an atom is empty.
- There is something in the centre of an atom called nucleus.
- Nucleus is positively charged.
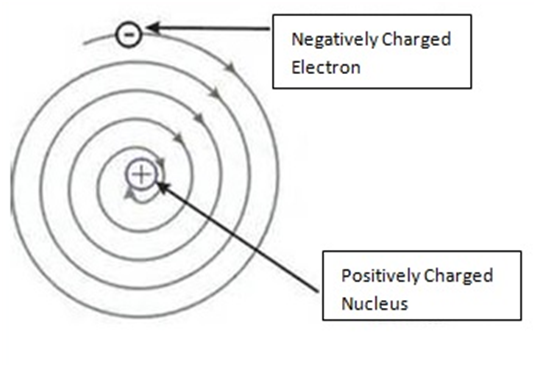
So, According To Rutherford, the structure of an atom is similar to that of the solar system.
He said:
- Atom is electrically neutral
- Nucleus is in centre in which protons are present.
- Outside nucleus electrons revolve like planets revolve around the Sun.
In this model also few limitations were seen and some questions were left unanswered, which led to its failure.
The drawback of Rutherford’s experiment were as follows –
He failed to explain the stability of an atom”.
According To electromagnetic theory: Any charged particle, when revolves in a circular path, continuously emits energy and shortens its path. As we know, an electron is also a charged particle revolving in a circular path so it should also emit energy, shorten its path and should finally falls into the nucleus and for doing so, it needs time lesser than a fraction of a second. But this doesn’t happen.

 Science Made Easy
Science Made Easy
 ACERISE INDIA
ACERISE INDIA
