- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Dalton's Atomic Theory
→ According to Dalton’s atomic theory, all matter, whether an element, a compound or a mixture is
composed of small particles called atoms.
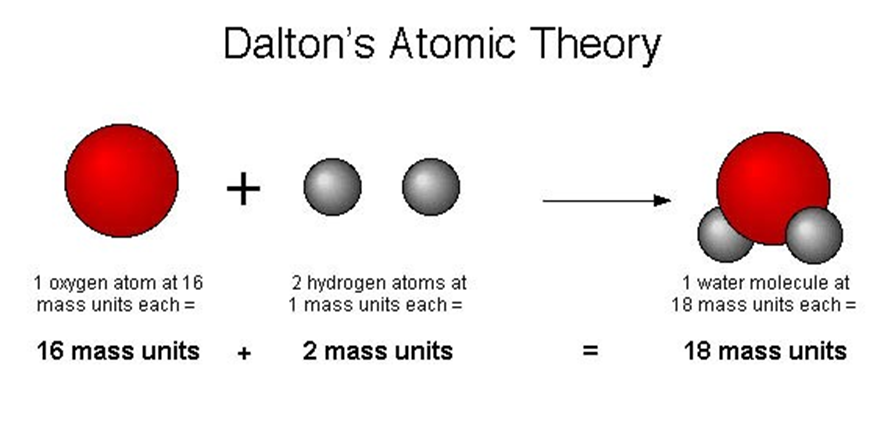
→ Six Postulates of Dalton's atomic theory:
(i) All matter is made of very tiny particles called atoms.
(ii) Atoms are indivisible particles, which cannot be created or destroyed in a chemical reaction.
(iii) Atoms of a given element are identical in mass and chemical properties. (Law of conservation of mass)
(iv) Atoms of different elements have different masses and chemical properties.
(v) Atoms combine in the ratio of small whole numbers to form compounds. (Law of constant proportion)
(vi) The relative number and kinds of atoms are constant in a given compound.

 Vaishnav Publication
Vaishnav Publication
 ACERISE INDIA
ACERISE INDIA
