- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
IUPAC and atomic Symbols
IUPAC and Atomic symbols
IUPAC – International Union Of Pure & Applied Chemistry
The IUPAC approves the names of elements. According to it,
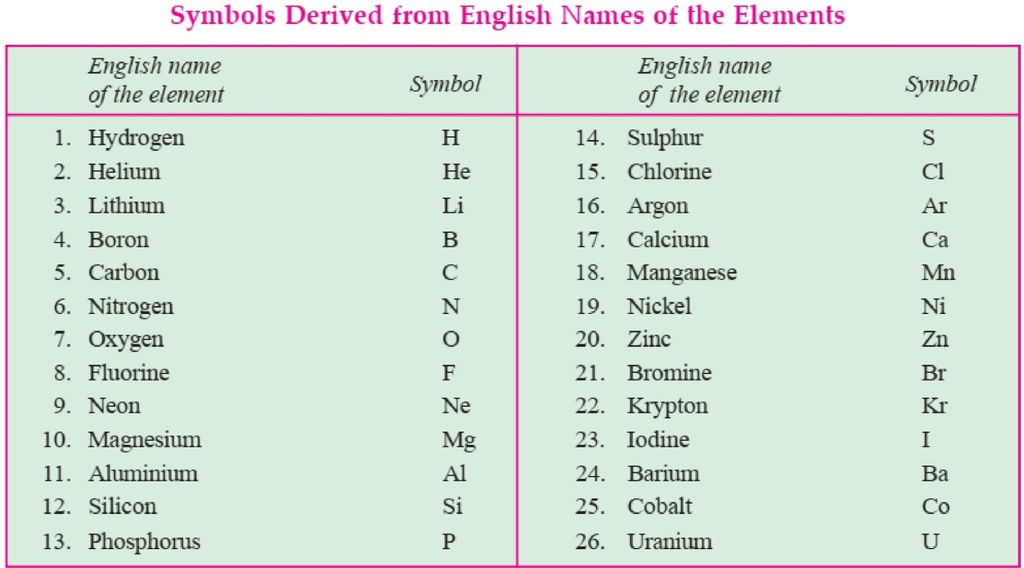
1. Symbol if an element is either the first letter of the name of the element or the first two letters of the name of an element.
2. In case there are two letters, then the first letter is in a capital case and the second letter is in small case.
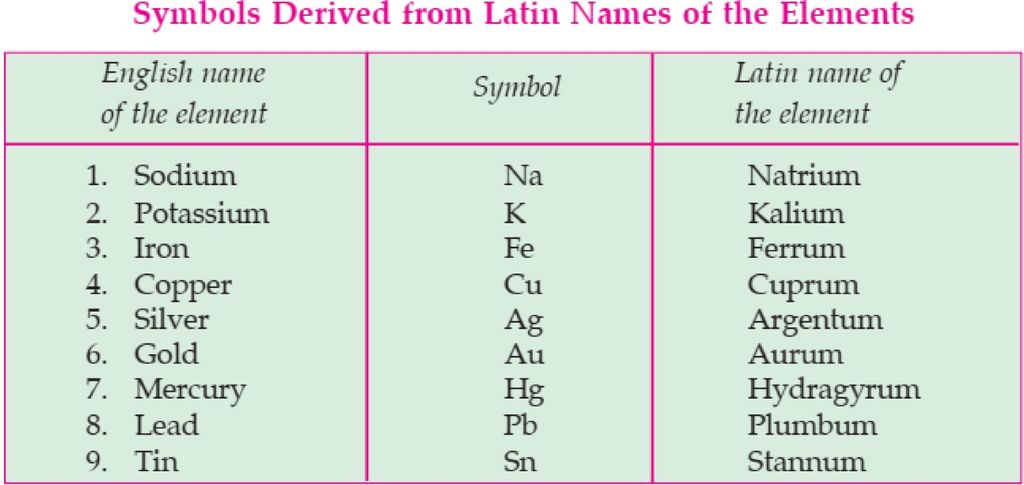
For example- lead is written as “Pb” and not as “pb”. Sometimes, the symbol is derived from the Latin name of an element. For example, if we look at the symbol of Sodium- “Na” and not .So, because it is derived from its Latin name that is, “Natrium”. Similarly, for Potassium, Copper, Iron, Mercury, etc. Latin names and symbols of a few elements are given below:
The atomic mass of the element
If we try to find the exact mass of an atom, we can’t find it. The reason being that an atom is too small in size that it is difficult to measure its size and moreover, the size of an atom changes, depending upon its neighbouring- atoms. Therefore, instead of finding its absolute mass, we try to find the relative mass of an atom. The standard element chosen for finding relative mass is carbon-6.
The relative atomic mass of an atom of an element is defined as the average relative mass of an atom as compared to an atom of 612c taken as 12u.
Atomic mass: In simple language, atomic mass is the number of times an atom of an element is heavier than 1/12th of a Carbon atom. For example: If we say that the atomic mass of Sodium is 23, it means that sodium is 23 times heavier than 1/12th of a carbon atom.
Molecule
The particle next to an atom is a molecule, but it has an independent existence and is formed when atoms combine with each other. Let us study about it. A Molecule is the smallest particle of an element or compound which is able to exist independently. For example, carbon dioxide molecule, hydrogen gas molecule, etc . But it has been seen that some molecules are formed by the combination of two atoms of the same kind like H2 and some may have two or more atoms of different kinds like H2O. This shows that in a molecule any number of atoms can be present and they may be of the same or different kinds.

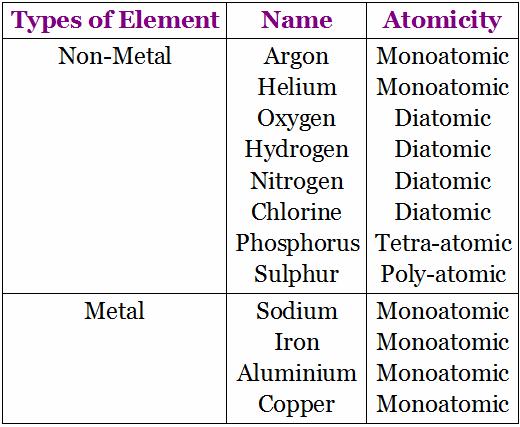
Atomicity: It is the number or the kinds of atoms present in a molecule of an element. Some more examples: Phosphorous exists as P4 (tetra atomic), Sulphur exists as S8 (polyatomic), Nitrogen exists as N2 (diatomic).

 Science Made Easy
Science Made Easy
 ACERISE INDIA
ACERISE INDIA
