1. Pure Substance
Is Matter around us pure
Introduction
When we talk about pure, it means that all the constituent particles of that substance are the same in their chemical nature. A pure substance consists of a single type of a particles. What is the type of pure substances?
- Elements
- Robert Boyle A was the first scientist to use the term element in 1661.
- Antoine Laurent Lavoisier (1743–94), a French chemist, was the first to establish an experimentally useful definition of an element.
- Elements can be normally divided into metals, non – metals and metalloids.
Metals
- Metals usually show some or all of the following properties:
- They have a lustre (shine).
- They conduct heat and electricity.
- They are ductile (can be drawn into wires).
- They are malleable (can be hammered into thin sheets).
- They are sonorous (make a ringing sound when hit).
# Examples of metals are gold, silver, copper, iron, sodium, potassium etc.
# Mercury is the only metal that is liquid at room temperature.
Non metals
- Non – metals usually show some or all of the following properties:
- They are poor conductors of heat and electricity.
- They are not lustrous, sonorous or malleable.
# Examples of non – metals are hydrogen. oxygen, iodine, carbon (coal, coke). bromine, chlorine etc.
Metalloids
- Metallaoids have intermediate properties between of metals and non – metals.
# Examples are boron, silicon, germanium etc.
Mixture and compound
| Mixture | Compound |
| 1. Elements or compounds are simple calling so new substance is formed. Compound | 1. Substances Are Reated Together with each other to make a new substance. |
| 2. Elements do not combine in a fixed ratio. | 2. Compositions the the component is Fixed i.e. , They combine together in a fixed ratio according to their masses. |
| 3. A mixture shows the properties of its components | 3. compound does not show the Properties of component elements. |
| 4. Components can be easily separated by any mechanical method which is suitable. | 4. components can not be separated from each other by simple mechanical methods. |
2. Impure Substance
But when we see around us, we observe most of the matter around us exists as mixtures of two or more pure components. For example: Sea water, Air etc.
WHAT IS A MIXTURE?
It is a form of matter in which two or more elements or compounds combine physically in any proportion by weight.
Characteristics of Mixture
- Mixture may be homogeneous and heterogeneous.
- Mixture does not have a fixed melting point.
- In a mixture, the different constituents combine physically in any proportion by mass.
- The constituents of a mixture do not lose their identical property.
- Usually, no energy change take place during the formation of a mixture.
Types of Mixtures
- Homogeneous mixture: A mixture which has same composition throughout. It has no visible boundaries of separation between the various constituents Solutions are homogeneous mixtures. For example, Detergent in water, Sugar in water, Ice cream etc.
- Heterogeneous mixture: A mixture which has different compositions in different parts. These types of mixtures have visible boundaries of separation between the various constituents. For example, Oil in water, Fruit salad, Sand in water etc.
3. Separation of Mixtures
WHAT IS SOLUTION?
A solution is a homogenous mixture of two or more substances. E.g. Nimboo pani, Soda water. A solution has a solvent and a solute as its components.
Solvent: The component of the solution that dissolves the other component in it is called the solvent.
Solute: The component of the solution that is dissolved is called the solute. in the solvent
Alloys: They are the mixtures of two or more metal or a metal and a non-metal and cannot be separated into their components by physical methods.
![]()
Properties of a Solution
• A Solution is a Homogeneous mixture.
• The particles of a solution are smaller than 1nm (10^-9 Metre) in Diameter. They cannot bean by Naked eyes.
• They are of very Small Particle Size, so they do not scatter a beam of lighting passing through the solution.
• The Solute Particles Cannot be separated from the mixture by the process of filtration.
Concentration of a solution
• Saturated solution: Depending upon the amount of solute present in a solution, it can be called a dilute, concentrated or a saturated solution.
• Unsaturated solution: If the amount of solute contained in a solution is less than the saturation level, it is called an unsaturated solution.
• Solubility: The amount of the solute present in the saturated solution at this temperature is called its solubility.
• The concentration of a solution is the amount of solute present in a given amount (mass or volume) of solution, or the amount of solute dissolved in a given mass or volume of solvent.
• Concentration of solution = Amount of solute / Amount of solution
Or
Amount of solute / Amount of solvent
Ways of expressing the concentration of a solution
• Mass by mass percentage of a solution
Mass of solute / Mass of solution x100
• Mass by volume percentage of a solution
Mass of solute / Volume of solution x100
4. Physical And Chemical Changes
What is a suspension?
In which solids are dispersed in liquids, are called suspensions.
A suspension is a heterogeneous mixture
Particles of a suspension are visible to the naked eye.
Properties of a Suspension
• Suspension is a heterogeneous mixture.
• The particles of a suspension can be seen by the naked eye.
- The particles of a suspension scatter a beam of light passing through it and make its path visible.
• The solute particles settle down when a suspension is left undisturbed, that is, a suspension is unstable.
WHAT IS A COLLOIDAL SOLUTION?
A colloidal solution is a heterogeneous mixture, for example, milk.
Because of the small size of colloidal particles, we cannot see them with naked eyes.
These particles can easily scatter a beam of visible light.
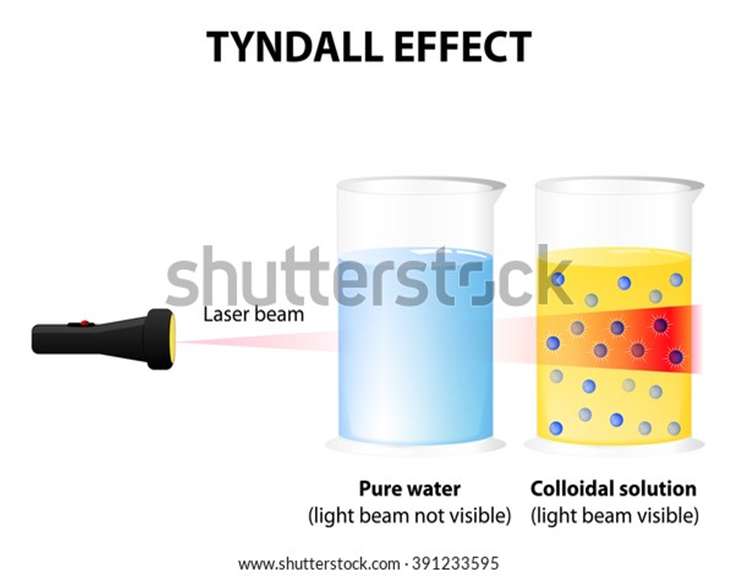
Tyndall effect
The scattering of a beam of light is called the Tyndall effect
The Tyndall effect can also be observed when a fine beam of light enters a room through a small hole.
This happens due to the scattering of light by the particles of dust and smoke in the air.
Observation of Tyndall effect
The Tyndall effect can be observed when sunlight passes through the canopy of a dense forest.
Properties of a colloid.
A colloid is a heterogeneous mixture.
The size of particles of a colloid is too small to be individually seen by naked eyes.
Colloids are large enough to scatter a beam of light passing through it and make its path visible.
They do not settle down when left undisturbed, that is, a colloid is quite stable.
They cannot be separated from the mixture by the process of filtration.
Dispersing medium
The components of a colloidal solution are the dispersed phase and the dispersion medium.
The solute – like component or the dispersed particles in a colloid form the dispersed phase, and the component in which the dispersed phase is suspended is known as the dispersing medium.

 Science Made Easy
Science Made Easy
 ACERISE INDIA
ACERISE INDIA
